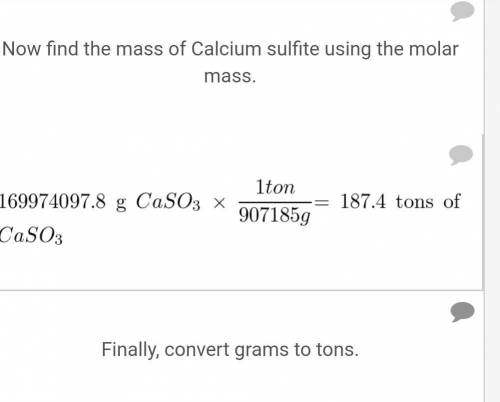
Chemistry, 20.02.2020 00:14 cheerleaderautumnche
A particular coal contains 2.5% sulfur by mass. When this coal is burned at a power plant, the sulfur is converted into sulfur dioxide gas, which is a pollutant. To reduce sulfur dioxide emissions, calcium oxide (lime) is used. The sulfur dioxide reacts with calcium oxide to form solid calcium sulfite. If the coal is burned in a power plant that uses 2000.0 tons of coal per day, what mass of calcium oxide is required daily to eliminate the sulfur dioxide?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 12:30
Which of the following describes a compound? (hint: carbon and oxygen bo a. a piece of pure carbon, containing only carbon atoms b. oxygen gas surrounding a solid piece of carbon c. a substance made of two oxygen atoms for each carbon atom carbon and oxygen atoms mixed without being bonded together
Answers: 1

Chemistry, 22.06.2019 13:00
Which of the following are good traits of a hypothesis? it will be able to be testedit can predict an outcomeit will explain the observationsall of these
Answers: 2

Chemistry, 22.06.2019 13:00
Imagine that you push on a large rock. at what point does your effort change the rock’s mechanical energy?
Answers: 1

Chemistry, 22.06.2019 14:00
How does the presence of oxygen affect the chemical pathways used to extract energy from glucose?
Answers: 3
You know the right answer?
A particular coal contains 2.5% sulfur by mass. When this coal is burned at a power plant, the sulfu...
Questions





Computers and Technology, 08.06.2020 19:57

Mathematics, 08.06.2020 19:57

Mathematics, 08.06.2020 19:57


Biology, 08.06.2020 19:57


Chemistry, 08.06.2020 19:57

Mathematics, 08.06.2020 19:57


Mathematics, 08.06.2020 19:57



Mathematics, 08.06.2020 19:57

English, 08.06.2020 19:57


English, 08.06.2020 19:57