
Chemistry, 20.02.2020 00:59 IzzybellaRamilo
What is the weight percent of vitamin C in a solution made by dissolving 6.50 g of vitamin C, C6H8O6, in 55.0 g of water?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 23:40
If the atomic mass of an atom is 34 and the atom contains 13 protons, how many neutrons does the atom contain?
Answers: 2

Chemistry, 22.06.2019 11:00
3) in peaches, [oh]=3.16x10-11 m a) find [h+ ] b) what is the ph? c) is the solution acidic, basic, or neutral?
Answers: 1

Chemistry, 22.06.2019 20:00
Phosphoric acid is a triprotic acid ( =6.9×10−3 , =6.2×10−8 , and =4.8×10−13 ). to find the ph of a buffer composed of h2po−4(aq) and hpo2−4(aq) , which p value should be used in the henderson–hasselbalch equation? p k a1 = 2.16 p k a2 = 7.21 p k a3 = 12.32 calculate the ph of a buffer solution obtained by dissolving 18.0 18.0 g of kh2po4(s) kh 2 po 4 ( s ) and 33.0 33.0 g of na2hpo4(s) na 2 hpo 4 ( s ) in water and then diluting to 1.00 l.
Answers: 3

Chemistry, 22.06.2019 22:00
All of the following are homogeneous mixtures except a) sugar dissolved in water. b) orange juice. c) coffee with cream. d) household vinegar. e) apple juice
Answers: 1
You know the right answer?
What is the weight percent of vitamin C in a solution made by dissolving 6.50 g of vitamin C, C6H8O6...
Questions

Mathematics, 18.03.2021 01:30

Physics, 18.03.2021 01:30

Health, 18.03.2021 01:30

Chemistry, 18.03.2021 01:30

Mathematics, 18.03.2021 01:30


Mathematics, 18.03.2021 01:30

English, 18.03.2021 01:30

English, 18.03.2021 01:30

Computers and Technology, 18.03.2021 01:30

Mathematics, 18.03.2021 01:30

Mathematics, 18.03.2021 01:30


Mathematics, 18.03.2021 01:30



Mathematics, 18.03.2021 01:30

Mathematics, 18.03.2021 01:30

Mathematics, 18.03.2021 01:30

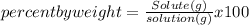 %
%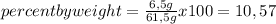 %
%

