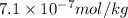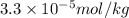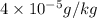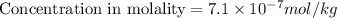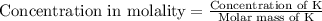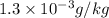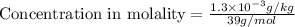Chemistry, 20.02.2020 01:51 khalilh1206
The concentrations of Fe and K in a sample of riverwater are 0.0400 mg/kg and 1.30 mg/kg, respectively. Express the concentration in molality.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:00
Fission of uranium-235 products energy and a. isotopes of smaller elements b. isotopes of larger elements c. lighter isotopes of uranium d. heavier isotopes of uranium
Answers: 3

Chemistry, 22.06.2019 10:30
Use this information to determine the number of calends electrons in the atoms. which of the following correctly compares the stability of the two atoms? a) both are unreactive b) both are highly reactive c) a is unreactive and d is reactive d) a is reactive and d is unreactive
Answers: 2

Chemistry, 22.06.2019 12:30
In france, grapes are 1.95 euros per kilogram. what is the cost of grapes, in dollars per pound, if the exchange rate is 1.14 dollars/euro? (2.6)
Answers: 3

Chemistry, 22.06.2019 14:30
Calculate the mass of carbon in 97.0 g of sucrose c12h22o11
Answers: 3
You know the right answer?
The concentrations of Fe and K in a sample of riverwater are 0.0400 mg/kg and 1.30 mg/kg, respective...
Questions


Mathematics, 30.04.2021 22:40

Mathematics, 30.04.2021 22:40

Mathematics, 30.04.2021 22:40

Biology, 30.04.2021 22:40



Mathematics, 30.04.2021 22:40


History, 30.04.2021 22:40



English, 30.04.2021 22:40

Mathematics, 30.04.2021 22:40

Mathematics, 30.04.2021 22:40




Mathematics, 30.04.2021 22:40

Biology, 30.04.2021 22:50