

Answers: 2
Another question on Chemistry



Chemistry, 22.06.2019 22:30
Rank the four gases (air, exhaled air, gas produced from from decomposition of h2o2, gas from decomposition of nahco3) in order of increasing concentration of co2
Answers: 1

Chemistry, 23.06.2019 05:00
Match the term to its description match term definition chemical energy a) internal energy caused by vibrations of atoms and molecules electrical energy b) electromagnetic energy that travels in waves radiant energy c) the movement of an electrical charge thermal energy d) potential energy stored in the bonds between atoms
Answers: 1
You know the right answer?
The specific heat capacity of silver is 0.235 J/g ∙ K. Its melting point is 962 °C, and its enthalpy...
Questions


Mathematics, 10.11.2020 20:50



Mathematics, 10.11.2020 20:50

Chemistry, 10.11.2020 20:50






Health, 10.11.2020 20:50

English, 10.11.2020 20:50



Arts, 10.11.2020 21:00

Mathematics, 10.11.2020 21:00

History, 10.11.2020 21:00


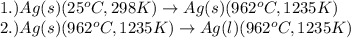
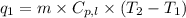
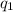 = amount of heat absorbed = ?
= amount of heat absorbed = ?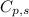 = specific heat capacity = 0.235 J/g.K
= specific heat capacity = 0.235 J/g.K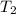 = final temperature = 1235 K
= final temperature = 1235 K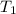 = initial temperature = 298 K
= initial temperature = 298 K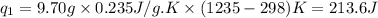
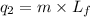
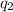 = amount of heat absorbed = ?
= amount of heat absorbed = ?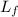 = latent heat of fusion = 11.3 kJ/mol =
= latent heat of fusion = 11.3 kJ/mol = 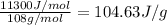 (Conversion factor: 1 kJ = 1000 J; Molar mass of silver = 108 g/mol)
(Conversion factor: 1 kJ = 1000 J; Molar mass of silver = 108 g/mol)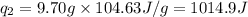
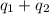
![[213.6+1014.9]J=1228.5J](/tpl/images/0516/5561/79110.png)


