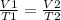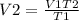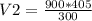Chemistry, 20.02.2020 05:24 puchie1225
A gas occupies 900.0 mL at a temperature of 27.0 ˚C. Assuming constant pressure, what is its volume if it is heated to 132.0 ˚C?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 09:00
At 300 mm hg, a gas has a volume of 380 l, what is the volume at standard pressure
Answers: 1

Chemistry, 22.06.2019 16:50
Answer asap need it by wednesday morning calculate the ph of 0.02m hcl best answer will be brainliest
Answers: 1


Chemistry, 23.06.2019 00:00
How is the way a mixture is combined different from how a compound is combined?
Answers: 3
You know the right answer?
A gas occupies 900.0 mL at a temperature of 27.0 ˚C. Assuming constant pressure, what is its volume...
Questions

History, 03.11.2019 01:31

Biology, 03.11.2019 01:31




English, 03.11.2019 01:31



Mathematics, 03.11.2019 01:31







Mathematics, 03.11.2019 01:31



Social Studies, 03.11.2019 01:31

Social Studies, 03.11.2019 01:31