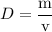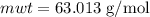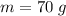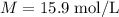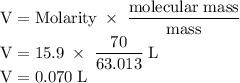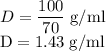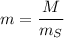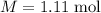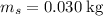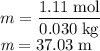Chemistry, 20.02.2020 03:38 anabelleacunamu
The concentration of commercially available concentrated nitric acid is 70.0 percent by mass, or 15.9 M. Calculate the density and molality of the solution.\

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 14:30
Amixture that has two or more substances that are spread out evenly is called a. compound b. heterogeneous c. substance d. homogeneous
Answers: 1

Chemistry, 22.06.2019 14:50
Consider the following multistep reaction: a b→ab(slow) a ab→a2b(fast)−−−−−−−−−−−−−−−−− 2a b→a2b(overall) based on this mechanism, determine the rate law for the overall reaction. express your answer in standard masteringchemistry format. for example, if the rate law is k[a]3[b]2 type k*[a]^3*[b]^2
Answers: 3


Chemistry, 22.06.2019 20:30
The activation energy for the reaction no2(g)+co2(g)⟶no(g)+co(g) is ea = 300 kj/mol and the change in enthalpy for the reaction is δh = -100 kj/mol . what is the activation energy for the reverse reaction?
Answers: 3
You know the right answer?
The concentration of commercially available concentrated nitric acid is 70.0 percent by mass, or 15....
Questions



Biology, 30.11.2020 20:30





History, 30.11.2020 20:30

Mathematics, 30.11.2020 20:30



Mathematics, 30.11.2020 20:30

English, 30.11.2020 20:30



Mathematics, 30.11.2020 20:30

History, 30.11.2020 20:30

History, 30.11.2020 20:30

Mathematics, 30.11.2020 20:30