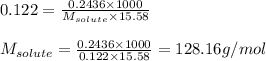Chemistry, 20.02.2020 03:33 cheerleader791
A. 0.2436 sample of an unknown substance was dissolved in 20.0mL of cyclohexane. The density of cyclohexane is 0.779 g/mL. The freezing point depression was 2.50 oC and the Kf value for cyclohecane is 20.5oC/m. Calculate the molality of the above solution and the molar mass of unknown.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 2


Chemistry, 23.06.2019 00:30
Ok, so i have 2 questions. try to answer them both: (the topic is fire) 1) how can you represent the chemical reaction of fire? 2) what kind of bond is formed in this chemical reaction
Answers: 3

Chemistry, 23.06.2019 08:00
If the solubility of a gas in water is 1.22 g/l at 2.75 atm, what is its solubility (in g/l) at 1.0 atm?
Answers: 1
You know the right answer?
A. 0.2436 sample of an unknown substance was dissolved in 20.0mL of cyclohexane. The density of cycl...
Questions

History, 12.03.2021 05:30


Mathematics, 12.03.2021 05:30


Mathematics, 12.03.2021 05:30



Mathematics, 12.03.2021 05:30



Mathematics, 12.03.2021 05:30

Mathematics, 12.03.2021 05:30

Biology, 12.03.2021 05:30




Mathematics, 12.03.2021 05:30

Mathematics, 12.03.2021 05:30

Mathematics, 12.03.2021 05:30

Mathematics, 12.03.2021 05:30

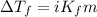
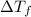 = depression in freezing point = 2.50°C
= depression in freezing point = 2.50°C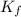 = molal freezing point elevation constant = 20.5°C/m
= molal freezing point elevation constant = 20.5°C/m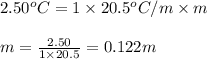
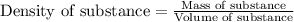
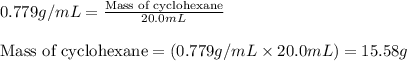
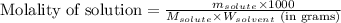
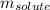 = Given mass of solute = 0.2436 g
= Given mass of solute = 0.2436 g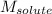 = Molar mass of solute = ? g/mol
= Molar mass of solute = ? g/mol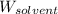 = Mass of solvent (cyclohexane) = 15.58 g
= Mass of solvent (cyclohexane) = 15.58 g