
Chemistry, 20.02.2020 03:31 thedoragreen
Theoretically, what mass of [Co(NH3)4(H2O)2]Cl2 could be produced from 4.00 g of CoCl2•6H2O starting material. If 1.20 g of [Co(NH3)4(H2O)2]Cl3 is produced, what is the percent yield?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:10
What approach is required to balance the objectives of sustainable development? balancing the objectives of sustainable development requires a(n) .
Answers: 3

Chemistry, 22.06.2019 03:30
Adrop of acetone (nail polish remover) has a mass of 35 mg and a density of 0.788 g/cm3. what is its volume in cubic centimeters?
Answers: 3

Chemistry, 22.06.2019 06:30
If 1.8 l of water is added to 2.5l of a 7.0 molarity koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 22.06.2019 11:00
What is the molar mass of a gas that has density of 2.054 g/l
Answers: 2
You know the right answer?
Theoretically, what mass of [Co(NH3)4(H2O)2]Cl2 could be produced from 4.00 g of CoCl2•6H2O starting...
Questions



Mathematics, 08.11.2019 17:31



Mathematics, 08.11.2019 17:31

History, 08.11.2019 17:31

Geography, 08.11.2019 17:31

Mathematics, 08.11.2019 17:31

Mathematics, 08.11.2019 17:31





Mathematics, 08.11.2019 17:31


Chemistry, 08.11.2019 17:31

Mathematics, 08.11.2019 17:31

Mathematics, 08.11.2019 17:31

Mathematics, 08.11.2019 17:31

![[Co(NH_3)_4(H_2O)_2]Cl_2](/tpl/images/0516/7064/2cb06.png) is 3.93 g and 30.53 % respectively
is 3.93 g and 30.53 % respectively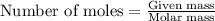 .....(1)
.....(1)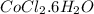 = 4.00 g
= 4.00 g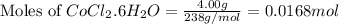
![CoCl_2.6H_2O+4NH_3\rightarrow [Co(NH_3)_4(H_2O)_2]Cl_2+4H_2O](/tpl/images/0516/7064/d38a0.png)
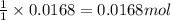 of
of ![0.0168mol=\frac{\text{Mass of }[Co(NH_3)_4(H_2O)_2]Cl_2}{234g/mol}\\\\\text{Mass of }[Co(NH_3)_4(H_2O)_2]Cl_2=(0.0168mol\times 234g/mol)=3.93g](/tpl/images/0516/7064/db933.png)
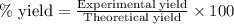
![\%\text{ yield of }[Co(NH_3)_4(H_2O)_2]Cl_2=\frac{1.20g}{3.93g}\times 100\\\\\% \text{yield of }[Co(NH_3)_4(H_2O)_2]Cl_2=30.53\%](/tpl/images/0516/7064/2c509.png)


