Chemistry, 20.02.2020 08:02 tahjaybenloss16
Consider the sodium borohydride reduction of camphor to isoborneol. A reaction was performed in which 1.400 1.400 g of camphor was reduced by an excess of sodium borohydride to make 1.040 1.040 g of isoborneol. Calculate the theoretical yield and percent yield for this reaction.

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 10:30
Consider the following reactions. (note: (s) = solid, (l) = liquid, and (g) = gas.) mg(s) + ½o2(g) → mgo(s) + 146 kcal/mole h2(g) + ½o2(g) → h2o(g), δh = -57.82 kcal/mole what type of reaction is represented by the previous two examples?
Answers: 3

Chemistry, 22.06.2019 17:40
How much heat is added if 0.814g of water increase in temperature by 0.351 degree c?
Answers: 3

You know the right answer?
Consider the sodium borohydride reduction of camphor to isoborneol. A reaction was performed in whic...
Questions


Biology, 22.01.2020 12:31

Biology, 22.01.2020 13:31

English, 22.01.2020 13:31

Mathematics, 22.01.2020 13:31

Biology, 22.01.2020 13:31





Mathematics, 22.01.2020 13:31



History, 22.01.2020 13:31

History, 22.01.2020 13:31

History, 22.01.2020 13:31

Mathematics, 22.01.2020 13:31

Computers and Technology, 22.01.2020 13:31

History, 22.01.2020 13:31

 .....(1)
.....(1)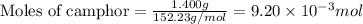
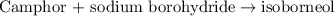
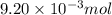 of camphor will produce =
of camphor will produce =  of isoborneol
of isoborneol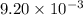 moles
moles