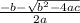Chemistry, 20.02.2020 08:37 thestuckone
At 700.°C, the total pressure of the system is found to be 3.01 atm. If the equilibrium constant KP is 1.52, calculate the equilibrium partial pressures of CO2 and CO.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:10
How do forces between particles in gases compare to forces in the other states of matter? o a. the forces in gases are stronger than forces in solids but weaker than forces in liquids. o b. the forces in gases are weaker than forces in solids but stronger than forces in liquids. o c. the forces in gases are weaker than forces in solids and liquids. o d. the forces in gases are stronger than forces in solids and liquids. submit
Answers: 1

Chemistry, 22.06.2019 01:30
When an object falls through the air and encounters air resistance its overall speed will be than if it had not encountered air resistance? (one word answer)
Answers: 2

Chemistry, 22.06.2019 06:30
The best solution for preventing harm to people and pets from severe hurricanes involves determining and warning residents about what
Answers: 1

Chemistry, 22.06.2019 23:00
What prefix multiplier is appropriate for reporting a measurement of 5.57 ×10−5 m?
Answers: 1
You know the right answer?
At 700.°C, the total pressure of the system is found to be 3.01 atm. If the equilibrium constant KP...
Questions


Mathematics, 31.08.2021 19:50

Social Studies, 31.08.2021 19:50


Chemistry, 31.08.2021 19:50

Advanced Placement (AP), 31.08.2021 19:50



World Languages, 31.08.2021 19:50

Physics, 31.08.2021 19:50

Physics, 31.08.2021 19:50


Mathematics, 31.08.2021 19:50

Engineering, 31.08.2021 19:50




English, 31.08.2021 19:50

Business, 31.08.2021 19:50

Mathematics, 31.08.2021 19:50

 = 1.5 atm
= 1.5 atm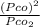
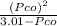 ⇒ 1.52 =
⇒ 1.52 = 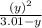 =
= 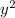 = 1.52 (3.01 - y)
= 1.52 (3.01 - y) 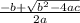 and y =
and y =