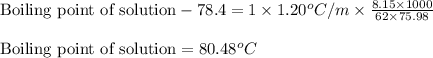Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 09:00
Acrystal that absorvd water from air is (blank)a. aqueousb. homogenousc. hygroscopicd. efflorescent
Answers: 1


Chemistry, 22.06.2019 16:30
For the reaction shown, calculate how many moles of no2 form when each of the following completely reacts. 2n2o5(g)→4no2(g)+o2(g) part a 1.0 mol n2o5 express your answer using two significant figures. nothing mol m o l request answer part b 5.4 mol n2o5 express your answer using two significant figures.
Answers: 2

Chemistry, 22.06.2019 19:50
A2.5% (by mass) solution concentration signifies that there is a 2.5 % (by mass) solution concentration signifies that there is blank of solute in every 100 g of solution. of solute in every 100 g of solution
Answers: 3
You know the right answer?
Calculate the freezing point and boiling point of a solution containing 8.15 g of ethylene glycol (C...
Questions


Biology, 22.07.2019 21:10





Social Studies, 22.07.2019 21:10

Mathematics, 22.07.2019 21:10

History, 22.07.2019 21:10





Mathematics, 22.07.2019 21:10


Mathematics, 22.07.2019 21:10

Mathematics, 22.07.2019 21:10

Biology, 22.07.2019 21:10


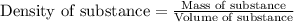
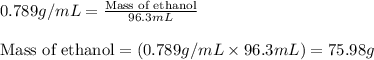
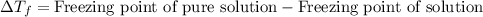
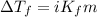
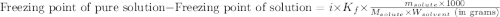
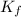 = molal freezing point elevation constant = 1.99°C/m
= molal freezing point elevation constant = 1.99°C/m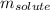 = Given mass of solute (ethylene glycol) = 8.15 g
= Given mass of solute (ethylene glycol) = 8.15 g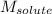 = Molar mass of solute (ethylene glycol) = 62 g/mol
= Molar mass of solute (ethylene glycol) = 62 g/mol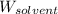 = Mass of solvent (ethanol) = 75.98 g
= Mass of solvent (ethanol) = 75.98 g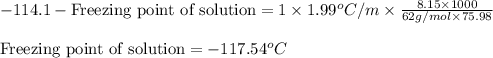
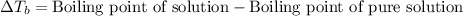
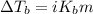
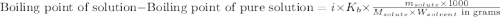
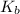 = molal boiling point elevation constant = 1.20°C/m.g
= molal boiling point elevation constant = 1.20°C/m.g