Chemistry, 20.02.2020 22:01 bartlettcs9817
Reaction is found to have a rate constant of 12.5 s-1 at 25.0 Celsius. When you heat the reaction up by ten degrees Celsius, the rate of the reaction exactly doubles. What is the activation energy for this reaction in kJ/mol?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:00
What forms when chemical reactions combine pollution with sunlight?
Answers: 1

Chemistry, 22.06.2019 08:30
What method(s) do plants use to obtain nitrogen? select all that apply. absorb it from the atmosphere use bacteria to convert nitrogen to usable form obtain usable nitrogen compounds from the soil absorb nitrogen from water taken in at the roots
Answers: 3

Chemistry, 22.06.2019 17:30
Oil rich countries in the middle east cover about 4% of earths total land area but prossess about 48% of the worlds known oil reserves what is the main reason for high concentration of reserves in this part of the world
Answers: 3

You know the right answer?
Reaction is found to have a rate constant of 12.5 s-1 at 25.0 Celsius. When you heat the reaction up...
Questions


Mathematics, 22.07.2021 20:20

Computers and Technology, 22.07.2021 20:20


History, 22.07.2021 20:20








Mathematics, 22.07.2021 20:20

Mathematics, 22.07.2021 20:20





Mathematics, 22.07.2021 20:20

English, 22.07.2021 20:20

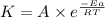
![\log (\frac{K_2}{K_1})=\frac{Ea}{2.303\times R}[\frac{1}{T_1}-\frac{1}{T_2}]](/tpl/images/0517/9661/6d953.png)
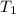 = initial temperature =
= initial temperature = 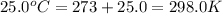
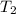 = final temperature =
= final temperature = 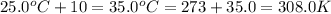
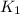 = rate constant at
= rate constant at 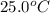 =
= 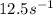
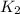 = rate constant at
= rate constant at 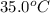 =
= 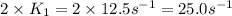
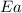 = activation energy for the reaction = ?
= activation energy for the reaction = ?![\log (\frac{25.0s^{-1}}{12.5s^{-1}})=\frac{Ea}{2.303\times 8.314J/mole.K}[\frac{1}{298.0K}-\frac{1}{308.0K}]](/tpl/images/0517/9661/6e305.png)