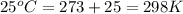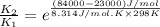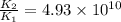Chemistry, 20.02.2020 22:23 electronia
The activation energy for a reaction is 84.0kJ/mol. Addition of a catalyst lowers the activation energy by 23.0 kJ/mol, while leaving the A-factor unchanged. By what factor ( uncatalyzed catalyzed k k ) does the rate increase at 25 °C?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 20:30
You are to give ampicillin with a recommended dose of 25mg/kg to a child with a mass of 29kg. if stock on hand is 250mg/capsule how many capsules should be given?
Answers: 1

Chemistry, 22.06.2019 03:10
Agas diffuses 1/7 times faster than hydrogen gas (h2). what is the molar mass of the gas? 100.10 g/mol 98.78 g/mol 86.68 g/mol 79.98 g/mol
Answers: 3

Chemistry, 22.06.2019 13:00
Asubstance is a good conductor of electricity which of the following best explains a probable position of the substance in a periodic table
Answers: 3

Chemistry, 22.06.2019 22:00
What mass of glucose is produced when 54g of water react with carbon dioxide
Answers: 1
You know the right answer?
The activation energy for a reaction is 84.0kJ/mol. Addition of a catalyst lowers the activation ene...
Questions



English, 16.07.2019 16:30

Mathematics, 16.07.2019 16:30



Physics, 16.07.2019 16:30

Mathematics, 16.07.2019 16:30


Biology, 16.07.2019 16:30




Mathematics, 16.07.2019 16:30

Biology, 16.07.2019 16:30

History, 16.07.2019 16:30

Mathematics, 16.07.2019 16:30

History, 16.07.2019 16:30


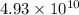
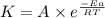
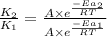
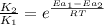
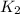 = rate of reaction with catalyst
= rate of reaction with catalyst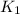 = rate of reaction without catalyst
= rate of reaction without catalyst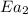 = activation energy with catalyst = 23.0 kJ/mol = 23000 J/mol
= activation energy with catalyst = 23.0 kJ/mol = 23000 J/mol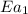 = activation energy without catalyst = 84.0 kJ/mol = 84000 J/mol
= activation energy without catalyst = 84.0 kJ/mol = 84000 J/mol