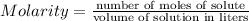Chemistry, 20.02.2020 22:27 mbrisen7420
You make 1.000 L of an aqueous solution that contains 35.0 g of ribose (C5H10O5). How many liters of water would you have to add to this solution to reduce the molarity you calculated in Part A (.233 moles) by a factor of two?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 07:20
2pos suppose an object in free fall is dropped from a building. its starting velocity is 0 m/s. ignoring the effects of air resistance, what is the speed (in m/s) of the object after falling 3 seconds? give your answer as a positive decimal without units. answer here
Answers: 1


Chemistry, 22.06.2019 11:30
What is the main reason why some developing countries fear the increase the free trade policies around the world?
Answers: 2

Chemistry, 22.06.2019 13:00
The number of neutrons is equal to the atomic number minus the atomic mass. a. true b. false
Answers: 2
You know the right answer?
You make 1.000 L of an aqueous solution that contains 35.0 g of ribose (C5H10O5). How many liters of...
Questions









Spanish, 01.09.2019 00:20


History, 01.09.2019 00:20



Business, 01.09.2019 00:20


Law, 01.09.2019 00:20




Mathematics, 01.09.2019 00:20