
Chemistry, 20.02.2020 22:41 cmflores3245
What is the vapor pressure of the solution if 35.0 g of water is dissolved in 100.0 g of ethyl alcohol at 25 ∘C? The vapor pressure of pure water is 23.8 mmHg, and the vapor pressure of ethyl alcohol is 61.2 mmHg at 25 ∘C.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 10:30
Use this information to determine the number of calends electrons in the atoms. which of the following correctly compares the stability of the two atoms? a) both are unreactive b) both are highly reactive c) a is unreactive and d is reactive d) a is reactive and d is unreactive
Answers: 2

Chemistry, 22.06.2019 18:30
The table lists the lattice energies of some compounds.compoundlattice energy (kj/mol)lif –1,036licl –853naf –923kf –821nacl –786which statement about crystal lattice energy is best supported by the information in the table? the lattice energy increases as cations get smaller, as shown by lif and kf.the lattice energy increases as the cations get larger, as shown by lif and licl.the lattice energy decreases as cations get smaller, as shown by nacl and naf.the lattice energy decreases as the cations get smaller, as shown by naf and kf.
Answers: 3

Chemistry, 22.06.2019 22:30
What is the work done by the electric force to move a 1 c charge from a to b?
Answers: 2

Chemistry, 23.06.2019 04:40
Temperature is defined as a. the equivalent of heat. b. a measure of the average kinetic energy of the individual atoms or molecules composing a substance. c. how hot or cold it is. d. the total kinetic energy of the atoms or molecules composing a substance. e. none of the above is correct.
Answers: 1
You know the right answer?
What is the vapor pressure of the solution if 35.0 g of water is dissolved in 100.0 g of ethyl alcoh...
Questions


Mathematics, 20.09.2020 17:01

Health, 20.09.2020 17:01

Mathematics, 20.09.2020 17:01


Social Studies, 20.09.2020 17:01

Advanced Placement (AP), 20.09.2020 17:01

Biology, 20.09.2020 17:01


Mathematics, 20.09.2020 17:01





Geography, 20.09.2020 17:01

History, 20.09.2020 17:01


History, 20.09.2020 17:01


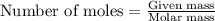 .....(1)
.....(1)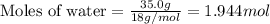
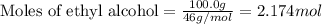
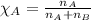
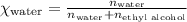
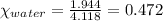
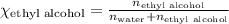
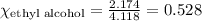

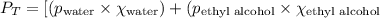
![p_T=[(23.8\times 0.472)+(61.2\times 0.528)]\\\\p_T=43.55mmHg](/tpl/images/0518/1070/83e05.png)


