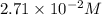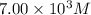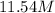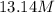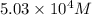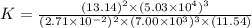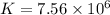Chemistry, 20.02.2020 23:42 corrineikerd
For this heterogeneous system 2A(aq)+3B(g)+C(l) ↽− −⇀ 2D(s)+3E(g) 2A(aq)+3B(g)+C(l)↽−−⇀2D(s)+3E(g) the concentrations and pressures at equilibrium are [A]=2.71× 10 −2 M [A]=2.71×10−2 M , P B =7.00× 10 3 Pa PB=7.00×103 Pa , [C]=11.54 M [C]=11.54 M , [D]=13.14 M [D]=13.14 M , and P E =5.03× 10 4 torr PE=5.03×104 torr . Calculate the thermodynamic equilibrium constant, K K .

Answers: 2
Another question on Chemistry


Chemistry, 21.06.2019 21:50
H2so4(aq) + mg(s)—> mgso4(aq) +h2(g) which substance is the acid in the reaction?
Answers: 3

Chemistry, 22.06.2019 04:20
Which of the following is true for the actual yield of a reaction? it is always calculated as a ratio. it is the yield from the excess reactant. it is the yield from the limiting reactant. it is always less than the theoretical yield.
Answers: 1

Chemistry, 22.06.2019 10:40
Asolid that forms and separates from a liquid mixture is called
Answers: 2
You know the right answer?
For this heterogeneous system 2A(aq)+3B(g)+C(l) ↽− −⇀ 2D(s)+3E(g) 2A(aq)+3B(g)+C(l)↽−−⇀2D(s)+3E(g) t...
Questions


Mathematics, 05.01.2021 01:00


Mathematics, 05.01.2021 01:00

Chemistry, 05.01.2021 01:00

English, 05.01.2021 01:00


English, 05.01.2021 01:00

Mathematics, 05.01.2021 01:00

English, 05.01.2021 01:00

History, 05.01.2021 01:00

Mathematics, 05.01.2021 01:00

Mathematics, 05.01.2021 01:00

Mathematics, 05.01.2021 01:00

Spanish, 05.01.2021 01:00

Mathematics, 05.01.2021 01:00



Mathematics, 05.01.2021 01:00

History, 05.01.2021 01:00

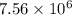
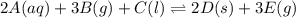
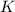 will be,
will be,![K=\frac{[D]^2[E]^3}{[A]^2[B]^3[C]}](/tpl/images/0518/2733/ab45f.png)