Chemistry, 20.02.2020 23:42 xxleeciexx
Use the ΔHrxn values of the following reactions: 2SO2(g) + O2(g) → 2SO3(g) ΔHrxn = –196 kJ 2S(s) + 3O2(g) → 2SO3(g) ΔHrxn = –790 kJ to calculate the ΔHrxn value of this reaction: S(s) + O2(g) → SO2(g) ΔHrxn = ?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 17:40
In the reading, yao chen-yuan describes traveling to deliver a message. why was he willing to risk danger to travelto tientsin? he wanted to the boxers with their cause
Answers: 2

Chemistry, 22.06.2019 07:00
6what is the importance of water on earth? a) it keeps the top layer of the geosphere cool b) it allows life to exist c) it provides ice at the poles d) it creates earth's blue color from space
Answers: 2

Chemistry, 22.06.2019 11:50
If oil spills continue, all of the following should be expected except (2 points) death of aquatic life. polluted groundwater. decreased soil productivity. increased global temperatures.
Answers: 3

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3
You know the right answer?
Use the ΔHrxn values of the following reactions: 2SO2(g) + O2(g) → 2SO3(g) ΔHrxn = –196 kJ 2S(s) + 3...
Questions





Social Studies, 07.12.2020 21:50


Chemistry, 07.12.2020 21:50



Chemistry, 07.12.2020 21:50










English, 07.12.2020 21:50

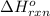 for the reaction is -297 kJ.
for the reaction is -297 kJ.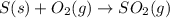
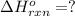
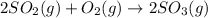
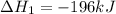
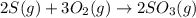
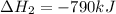
![\Delta H^o_{rxn}=\frac{[1\times (-\Delta H_1)]+[1\times \Delta H_2]}{2}](/tpl/images/0518/2732/98145.png)