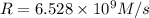Chemistry, 21.02.2020 00:00 ylianafghgfdsnm1479
The iodide ion reacts with hypochlorite ion (the active ingredient in chlorine bleaches) in the following way:
OCl−+I−→OI−+Cl−.
This rapid reaction gives the following rate data:
[OCl−](M) [I]−(M) Rate (M/s)
1.5×10^−3 1.5×10^−3 1.36×10^−4
3.0×10^−3 1.5×10^−3 2.72×10^−4
1.5×10^−3 3.0×10^−3 2.72×10^−4
a. Write the rate law for this reaction.
b. Calculate the rate constant with proper units.
c. Calculate the rate when [OCl-]= 1.8×10^3 M and [I-]= 6.0×10^4 M .

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 04:30
Why are people not able to scuba dive in the deep part of the ocean
Answers: 2

Chemistry, 22.06.2019 17:10
Some liquids can be distilled, but only at temperatures that are so high that it is impractical, or so high the compound decomposes. explain why distillation such compounds at significantly less than atmospheric pressure (some degree of vacuum) would solve this problem.
Answers: 2


Chemistry, 22.06.2019 22:30
Astudent pours 10.0 g of salt into a container of water and observes the amount of time it takes for the salt to dissolve. she then repeats the process using the same amounts of salt and water but this time she slowly stirs the mixture while it is dissolving. the student performs the experiment one more time but this time she stirs the mixture rapidly. the dependent variable in this experiment is: time for salt to dissolve speed of stirring amount of water mass of salt
Answers: 1
You know the right answer?
The iodide ion reacts with hypochlorite ion (the active ingredient in chlorine bleaches) in the foll...
Questions



Mathematics, 08.02.2021 23:40

Biology, 08.02.2021 23:40





Biology, 08.02.2021 23:40

Mathematics, 08.02.2021 23:40


Chemistry, 08.02.2021 23:40

History, 08.02.2021 23:40


Mathematics, 08.02.2021 23:40



English, 08.02.2021 23:40


![R=k[OCl^-]^1\times [I^-]^1](/tpl/images/0518/3250/d583c.png)
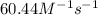 .
.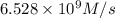 .
.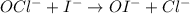
![R=k[OCl^-]^x\times [I^-]^y](/tpl/images/0518/3250/04fdb.png)
![[OCl^-]=1.5\times 10^{-3} M](/tpl/images/0518/3250/b77da.png) and
and ![[I^-]=1.5\times 10^{-3} M](/tpl/images/0518/3250/2f2e0.png) .
.![1.36\times 10^{-4} M/s=k[1.5\times 10^{-3} M]^x\times [1.5\times 10^{-3} M]^y](/tpl/images/0518/3250/e45d8.png) ..[1]
..[1]![[OCl^-]=3.0\times 10^{-3} M](/tpl/images/0518/3250/3317d.png) and
and ![2.72\times 10^{-4}M/s=k[3.0\times 10^{-3} M]^x\times [1.5\times 10^{-3} M]^y](/tpl/images/0518/3250/dea0f.png) ..[2]
..[2]![[I^-]=3.0\times 10^{-3} M](/tpl/images/0518/3250/b24ad.png) .
.![2.72\times 10^{-4} M/s=k[1.5\times 10^{-3} M]^x\times [3.0\times 10^{-3} M]^y](/tpl/images/0518/3250/22116.png) ..[3]
..[3]![\frac{1.36\times 10^{-4}M/s}{2.72\times 10^{-4}M/s}=\frac{k[1.5\times 10^{-3} M]^x\times [1.5\times 10^{-3} M]^y}{k[3.0\times 10^{-3} M]^x\times [1.5\times 10^{-3} M]^y}](/tpl/images/0518/3250/ebd3d.png)
![\frac{1.36\times 10^{-4} M/s}{2.72\times 10^{-4} M/s}=\frac{k[1.5\times 10^{-3} M]^x\times [1.5\times 10^{-3} M]^y}{k[1.5\times 10^{-3} M]^x\times [3.0\times 10^{-3} M]^y}](/tpl/images/0518/3250/11fc2.png)
![1.36\times 10^{-4} M/s=k[1.5\times 10^{-3} M]\times [1.5\times 10^{-3} M]](/tpl/images/0518/3250/f3ed7.png)
![k=\frac{1.36\times 10^{-4} M/s}{[1.5\times 10^{-3} M]\times [1.5\times 10^{-3} M]}=60.44 M^{-1}s^{-1}](/tpl/images/0518/3250/1ed07.png)
![[OCl^-]=1.8\times 10^{3} M](/tpl/images/0518/3250/b9482.png) and
and ![[I^-]=6.0\times 10^{4} M](/tpl/images/0518/3250/ceced.png) be R.
be R.![R=60.44 M^{-1}s^{-1}\times [1.8\times 10^{3} M]^1\times [6.0\times 10^{4} M]^1](/tpl/images/0518/3250/1384b.png)