
Please use the values in the resources listed below instead of the textbook values. Under certain conditions the decomposition of ammonia on a metal surface gives the following data.[NH3] (M) 2.0 ✕ 10−3 4.0 ✕ 10−3 6.0 ✕ 10−3 Rate (mol/L/h) 1.5 ✕ 10−6 1.5 ✕ 10−6 1.5 ✕ 10−6 Determine the rate equation for this reaction. (Rate expressions take the general form: rate = k . [A]a . [B]b.)

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 21:30
The density of an unknown gas at 98°c and 740 mmhg is 2.50 g/l. what is the molar mass of the gas with work showed?
Answers: 1

Chemistry, 22.06.2019 00:00
For ai it's atomic number is 13 and it's mass number is 27 how many neutrons does it have
Answers: 1

Chemistry, 22.06.2019 05:50
What happens when the temperature of a solution increases?
Answers: 2

You know the right answer?
Please use the values in the resources listed below instead of the textbook values. Under certain co...
Questions

Mathematics, 23.11.2020 01:50



Health, 23.11.2020 01:50

Mathematics, 23.11.2020 01:50

Chemistry, 23.11.2020 01:50


Mathematics, 23.11.2020 01:50

Mathematics, 23.11.2020 01:50

Computers and Technology, 23.11.2020 01:50

Mathematics, 23.11.2020 01:50



Mathematics, 23.11.2020 01:50

History, 23.11.2020 01:50

Mathematics, 23.11.2020 01:50

Biology, 23.11.2020 01:50

English, 23.11.2020 01:50


![R=k[NH_3]^0](/tpl/images/0518/3680/fda84.png)
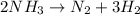
![R=k[NH_3]^x](/tpl/images/0518/3680/194a4.png)
![[NH_3]=2.0\times 10^{-3} M](/tpl/images/0518/3680/fed44.png)
![1.5\times 10^{-6} M/s=k[2.0\times 10^{-3} M]^x](/tpl/images/0518/3680/2ac63.png) ..[1]
..[1]![[NH_3]=4.0\times 10^{-3} M](/tpl/images/0518/3680/8a2bc.png)
![1.5\times 10^{-6} M/s=k[4.0\times 10^{-3} M]^x](/tpl/images/0518/3680/49a41.png) ..[2]
..[2]![\frac{1.5\times 10^{-6}M/s}{1.5\times 10^{-6}M/s}=\frac{k[2.0\times 10^{-3}M]^x}{k[4.0\times 10^{-3}M]^x}](/tpl/images/0518/3680/c6074.png)


