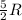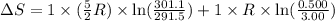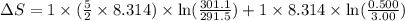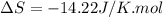Chemistry, 21.02.2020 01:05 AsiaDeas4078
During the test of an internal combustion engine, 3.00 L of nitrogen gas at 18.5 °C was compressed suddenly (and irrevers- ibly) to 0.500 L by driving in a piston. In the process, the tempera- ture of the gas increased to 28.1°C. Assume ideal behavior, what is the change in entropy of the gas?

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 12:30
In france, grapes are 1.95 euros per kilogram. what is the cost of grapes, in dollars per pound, if the exchange rate is 1.14 dollars/euro? (2.6)
Answers: 3

Chemistry, 22.06.2019 21:20
One way in which the useful metal copper is produced is by dissolving the mineral azurite, which contains copper(ii) carbonate, in concentrated sulfuric acid. the sulfuric acid reacts with the copper(ii) carbonate to produce a blue solution of copper(ii) sulfate. scrap iron is then added to this solution, and pure copper metal precipitates out because of the following chemical reaction: (s) (aq) (s) (aq) suppose an industrial quality-control chemist analyzes a sample from a copper processing plant in the following way. he adds powdered iron to a copper(ii) sulfate sample from the plant until no more copper will precipitate. he then washes, dries, and weighs the precipitate, and finds that it has a mass of .
Answers: 2

Chemistry, 22.06.2019 22:10
Which aqueous solution of ki freezes at the lowest temperature? 1) 1 mol of ki in 500. g of water 2) 2 mol of ki in 500. g of water 3) 1 mol of ki in 1000. g of water 4) 2 mol of ki in 1000. g of water
Answers: 3
You know the right answer?
During the test of an internal combustion engine, 3.00 L of nitrogen gas at 18.5 °C was compressed s...
Questions


Health, 15.12.2020 16:50

Mathematics, 15.12.2020 16:50

English, 15.12.2020 16:50


Mathematics, 15.12.2020 16:50






Biology, 15.12.2020 16:50








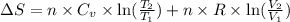
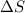 = change in molar entropy
= change in molar entropy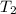 = final temperature =
= final temperature = 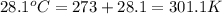
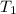 = initial temperature =
= initial temperature = 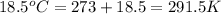
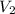 = final volume = 0.500 L
= final volume = 0.500 L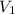 = initial volume = 3.00 L
= initial volume = 3.00 L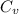 = heat capacity diatomic gas
= heat capacity diatomic gas 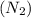 =
=