Chemistry, 21.02.2020 01:25 rainbowsauxe
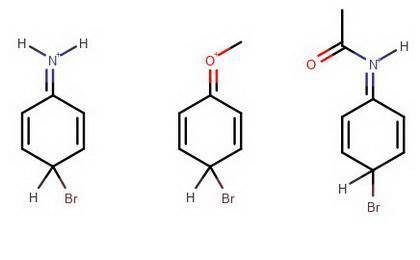
Draw the most stable resonance structure for the intermediate in the electrophilic aromatic bromination of aniline, anisole, and acetanilide to make the respective monobrominated products. compare their relative stability and the predict the relative reactivities of each starting compound.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 02:50
Consider the equilibrium system: 2icl(s) ⇄ i2(s) + cl2(g) which of the following changes will increase the total amount of of cl2 that can be produced? all of the listed answers are correct decreasing the volume of the container removing the cl2 as it is formed adding more icl(s) removing some of the i2(s)
Answers: 1

Chemistry, 22.06.2019 14:00
How is the atomic number of a nucleus changed by alpha decay
Answers: 2

Chemistry, 22.06.2019 17:00
The arrangement of particles is most ordered in a sample of
Answers: 1

Chemistry, 23.06.2019 01:30
Which is an example of a highly unstable isotope that is often used in fission reactions?
Answers: 1
You know the right answer?
Draw the most stable resonance structure for the intermediate in the electrophilic aromatic brominat...
Questions

Mathematics, 19.01.2021 14:00

History, 19.01.2021 14:00

English, 19.01.2021 14:00

Computers and Technology, 19.01.2021 14:00

Mathematics, 19.01.2021 14:00

Mathematics, 19.01.2021 14:00

Chemistry, 19.01.2021 14:00

Chemistry, 19.01.2021 14:00

History, 19.01.2021 14:00

Mathematics, 19.01.2021 14:00

Mathematics, 19.01.2021 14:00


English, 19.01.2021 14:00

Mathematics, 19.01.2021 14:00

History, 19.01.2021 14:00

Mathematics, 19.01.2021 14:00

Social Studies, 19.01.2021 14:00

Chemistry, 19.01.2021 14:00

History, 19.01.2021 14:00