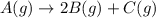Chemistry, 21.02.2020 01:15 ToonGamesToo
Consider the generic reaction: A (g) → 2 B (g) + C (g) . If a flask initially contains only A and the reaction proceeds to completion with a final pressure of 38.9 atm , what is the partial pressure of B in the container? (Assume constant volume and temperature.)

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 09:00
How are isotopes of the same chemical element alike? how are they different?
Answers: 1

Chemistry, 22.06.2019 22:10
Which aqueous solution of ki freezes at the lowest temperature? 1) 1 mol of ki in 500. g of water 2) 2 mol of ki in 500. g of water 3) 1 mol of ki in 1000. g of water 4) 2 mol of ki in 1000. g of water
Answers: 3

Chemistry, 23.06.2019 00:00
Before it was launched, a helium-filled balloon had a pressure of 201 kpa at a temperature of 27°c. at an altitude of 15,000 m, the pressure had decreased to 2.5 kpa and the temperature had dropped to -14 °c. the volume of the balloon increased to 59.3 m3. what is the original volume of the balloon? 13 m3 0.85 m3 0.077 m3 1.17 m3
Answers: 3
You know the right answer?
Consider the generic reaction: A (g) → 2 B (g) + C (g) . If a flask initially contains only A and th...
Questions


Computers and Technology, 20.10.2021 14:00



Computers and Technology, 20.10.2021 14:00


Mathematics, 20.10.2021 14:00





History, 20.10.2021 14:00





Physics, 20.10.2021 14:00



Computers and Technology, 20.10.2021 14:00