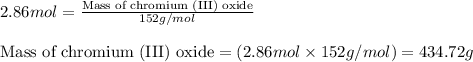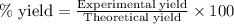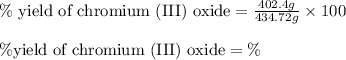Chromium(III) oxide can be prepared by heating chromium(IV) oxide in vacuo at high temperature: 4Cr02 —2Cr2O3 +02 The reaction of 480.1 g of CrO2 yields 402.4 g of Cr203. Calculate the theoretical yield of Cr203 (assuming complete reaction) and its percentage yield. Theoretical yield = Percentage yield = Submit Answer 2 question attempts remaining

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 14:30
Janel’s class studied properties of matter and how matter can change. janel decided she would do an experiment mixing baking soda and vinegar.question: describe the properties of baking soda and vinegar, and explain the changes that janel should see when she mixes the two types of matter. •first, identify the physical state of matter of baking soda. describe another property of baking soda. •next, identify the physical state of matter of vinegar. describe another property of vinegar. •then, explain what janel should see when she mixes the baking soda and vinegar. •describe the states of matter of the new materials that are formed. •explain how janel can be certain a change has occurred. me
Answers: 3

Chemistry, 22.06.2019 19:30
Draw the lewis structure for the trisulfur s3 molecule. be sure to include all resonance structures that satisfy the octet rule.
Answers: 3

Chemistry, 23.06.2019 02:10
Detrimental the length of the object shown 1. 97.8mm 2. 97.80 mm 3. 97mm 4. 98mm
Answers: 2

Chemistry, 23.06.2019 03:00
Abaker touches a pie right after taking it out of the oven. which statement best explains why the pie feels hot?
Answers: 1
You know the right answer?
Chromium(III) oxide can be prepared by heating chromium(IV) oxide in vacuo at high temperature: 4Cr0...
Questions

Biology, 27.04.2021 19:40


Mathematics, 27.04.2021 19:40

Law, 27.04.2021 19:40


SAT, 27.04.2021 19:40

English, 27.04.2021 19:40

Mathematics, 27.04.2021 19:40

Mathematics, 27.04.2021 19:40

Mathematics, 27.04.2021 19:40

Mathematics, 27.04.2021 19:40




Physics, 27.04.2021 19:40


English, 27.04.2021 19:40



Chemistry, 27.04.2021 19:40

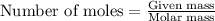 .....(1)
.....(1)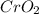 = 480.1 g
= 480.1 g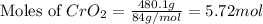
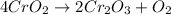
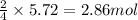 of chromium (III) oxide
of chromium (III) oxide