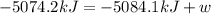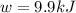Chemistry, 21.02.2020 02:32 smusisca53
The change in internal energy for the combustion of 1.0 mol of octane at a pressure of 1.0 atm is -5084.1 kJ. You may want to reference (Pages 381 - 385) Section 9.6 while completing this problem.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:00
If you add 10ml of hot water to 10ml of cold water and the change in tempature 8°c calculate how much energy is gained by the cold water
Answers: 1

Chemistry, 22.06.2019 06:00
What type of electromagnetic radiation has a shorter wavelength than blue light
Answers: 2

Chemistry, 22.06.2019 17:40
Areaction in which products can react to re-form reactants is
Answers: 1

Chemistry, 22.06.2019 18:30
Which of the following words describe the reality that the universe looks the same from various perspective
Answers: 3
You know the right answer?
The change in internal energy for the combustion of 1.0 mol of octane at a pressure of 1.0 atm is -5...
Questions

Mathematics, 25.05.2021 15:20

Chemistry, 25.05.2021 15:20


English, 25.05.2021 15:20


Mathematics, 25.05.2021 15:20

Mathematics, 25.05.2021 15:20

Business, 25.05.2021 15:20

Mathematics, 25.05.2021 15:20

Mathematics, 25.05.2021 15:20



English, 25.05.2021 15:20

Computers and Technology, 25.05.2021 15:20

Mathematics, 25.05.2021 15:20




Chemistry, 25.05.2021 15:20

Mathematics, 25.05.2021 15:20


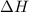 = change in enthalpy = -5074.2 kJ
= change in enthalpy = -5074.2 kJ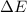 = change in internal energy = -5084.1 kJ
= change in internal energy = -5084.1 kJ