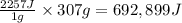Chemistry, 21.02.2020 02:28 akatian55721
As an athlete exercises, sweat is produced and evaporated to help maintain a proper body temperature. On average, an athlete loses approximately 307 g of sweat during an hour of exercise. How much energy is needed to evaporate the sweat that is produced? The heat of vaporization for water is 2257J/g. energy required:

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 01:00
According to the tide table below what time of day will the highest tide occur?
Answers: 1


Chemistry, 22.06.2019 04:40
In which environment would primary succession occur? a forest with a few remaining trees after a recent wildfire an area of exposed rock after a glacier melts away beach that is exposed to the air at low tide an abandoned baseball field in a small town
Answers: 1

Chemistry, 22.06.2019 05:30
Transportation is the largest single source of air pollution in the united states. air pollution can harm the environment and human health. which technology could offer a solution to this problem? mufflers that reduce noise motors that run on electricity tires that improve gas mileage
Answers: 3
You know the right answer?
As an athlete exercises, sweat is produced and evaporated to help maintain a proper body temperature...
Questions

Mathematics, 22.10.2020 23:01


Social Studies, 22.10.2020 23:01

Mathematics, 22.10.2020 23:01


Mathematics, 22.10.2020 23:01





Mathematics, 22.10.2020 23:01

English, 22.10.2020 23:01


History, 22.10.2020 23:01


History, 22.10.2020 23:01

Mathematics, 22.10.2020 23:01

Geography, 22.10.2020 23:01

Mathematics, 22.10.2020 23:01