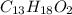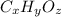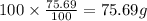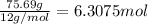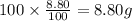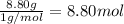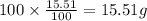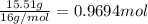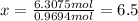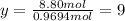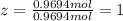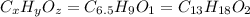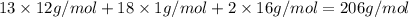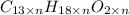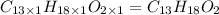Chemistry, 21.02.2020 02:26 sergun252005
Determine the empirical and molecular formulas of each of the following substances. For example, butane has an empirical formula of C2H5 (lowest whole-number ratio) and a molecular formula of C4H10, where the molecular formula corresponds to the molar mass of 58.12 g/mol.
Ibuprofen, a headache remedy, contains 75.69% C, 8.80% H, and 15.51% O by mass and has a molar mass of 206 g/mol.

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1

Chemistry, 23.06.2019 00:30
What is bromine+calcium iodide--> calcium bromide +iodine balanced
Answers: 1

Chemistry, 23.06.2019 03:30
Which of the following describes the entropy change as a solution is made from a liquid and solid
Answers: 1
You know the right answer?
Determine the empirical and molecular formulas of each of the following substances. For example, but...
Questions


Mathematics, 26.02.2021 20:30


Mathematics, 26.02.2021 20:30



Mathematics, 26.02.2021 20:30

Social Studies, 26.02.2021 20:30


Mathematics, 26.02.2021 20:30

Mathematics, 26.02.2021 20:30


Mathematics, 26.02.2021 20:30


Mathematics, 26.02.2021 20:30


Arts, 26.02.2021 20:30


Mathematics, 26.02.2021 20:30

Mathematics, 26.02.2021 20:30