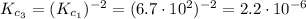Chemistry, 21.02.2020 02:21 bluefish743
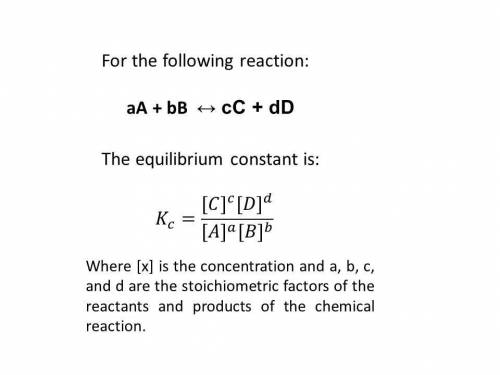
Be sure to answer all parts. Enter your answers in scientific notation. At a particular temperature, Kc = 6.7 × 102 for 2NO(g) + 2H2(g) ⇌ N2(g) + 2H2O(g) Calculate Kc for each of the following reactions:(a) NO(g) + H2(g)1/2 N2(g) + H2O(g)
(b) 2 N2(g) + 4 H2O(g)4 NO(g) + 4 H2(g)

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:40
How many electrons does silver have to give up in order to achieve a sido noble gas electron configuration?
Answers: 3

Chemistry, 22.06.2019 01:30
Idon't really understand this can you me and show your work.☺☺[ chemistry b] subject [ electron transfer in lonic bonds]grade( 12)
Answers: 1

Chemistry, 22.06.2019 12:00
An atom's configuration based on its number of electrons ends at 3p4. another atom has seven more electrons. starting at 3p, what is the remaining configuration? 3p63d34s2 3p43d54s2 3p64s23d3 3p44s23d
Answers: 3

Chemistry, 22.06.2019 14:20
Which statement explains why the bonds between non metals tend to be covalent? the bonds are found to be nondirectional they have large differences in electronegativity they have small differences in electronegativity they have ions that produce an electrostatic pull
Answers: 1
You know the right answer?
Be sure to answer all parts. Enter your answers in scientific notation. At a particular temperature,...
Questions



Business, 25.07.2019 15:00





Mathematics, 25.07.2019 15:00



History, 25.07.2019 15:00


History, 25.07.2019 15:00





History, 25.07.2019 15:00

Mathematics, 25.07.2019 15:00

![K_{c_{1}} = \frac{[N_{2}][H_{2}O]^{2}}{[NO]^{2}[H_{2}]^{2}} = 6.7 \cdot 10^{2}](/tpl/images/0518/6004/5a191.png) (2)
(2) ![K_{c_{2}} = \frac{[N_{2}]^{1/2}[H_{2}O]}{[NO][H_{2}]}](/tpl/images/0518/6004/75dc6.png) (4)
(4) ![\frac{[N_{2}]^{1/2}[H_{2}O]}{[NO][H_{2}]} = (\frac{[N_{2}][H_{2}O]^{2}}{[NO]^{2}[H_{2}]^{2}})^{1/2}](/tpl/images/0518/6004/6139f.png)
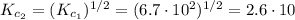
![K_{c_{3}} = \frac{[NO]^{4}[H_{2}]^{4}}{[N_{2}]^{2}[H_{2}O]^{4}}](/tpl/images/0518/6004/de89d.png) (6)
(6) ![\frac{[NO]^{4}[H_{2}]^{4}}{[N_{2}]^{2}[H_{2}O]^{4}} = \left(\frac{[N_{2}][H_{2}O]^{2}}{[NO]^{2}[H_{2}]^{2}}\right)^{-1}](/tpl/images/0518/6004/bc59e.png)
![\frac{[NO]^{4}[H_{2}]^{4}}{[N_{2}]^{2}[H_{2}O]^{4}} = \left(\frac{[NO]^{2}[H_{2}]^{2}}{[N_{2}][H_{2}O]^{2}}\right)^{2}](/tpl/images/0518/6004/80e13.png)