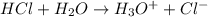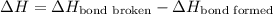Chemistry, 21.02.2020 02:31 chanelandme123
The O-H bond energy is 464 kJ/mol and the HI bond energy is 291 kJ/mol. Remembering that breaking any bond is an endothermic process and making any bond is exothermic, what is the net enthalpy change involved in the reaction between HI and H2O? Is this reaction endothermic or exothermic?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 03:30
Calculate the molar mass of aluminum oxide (al2o3). express your answer to four significant figures.
Answers: 1


Chemistry, 22.06.2019 10:30
Consider the following reactions. (note: (s) = solid, (l) = liquid, and (g) = gas.) mg(s) + ½o2(g) → mgo(s) + 146 kcal/mole h2(g) + ½o2(g) → h2o(g), δh = -57.82 kcal/mole what type of reaction is represented by the previous two examples?
Answers: 3

Chemistry, 22.06.2019 22:30
Which process describes vaporization that takes place below the surface of a liquid? condensation melting boiling evaporation
Answers: 1
You know the right answer?
The O-H bond energy is 464 kJ/mol and the HI bond energy is 291 kJ/mol. Remembering that breaking an...
Questions



Mathematics, 22.08.2019 15:10



Spanish, 22.08.2019 15:10

Mathematics, 22.08.2019 15:10

English, 22.08.2019 15:10

Physics, 22.08.2019 15:10

English, 22.08.2019 15:10


Mathematics, 22.08.2019 15:10

Mathematics, 22.08.2019 15:10

Mathematics, 22.08.2019 15:10


Mathematics, 22.08.2019 15:10

Biology, 22.08.2019 15:10



Chemistry, 22.08.2019 15:10