
Chemistry, 21.02.2020 03:00 darenl4478
The Fe 2 + ( 55.845 g/mol) content of a 2.349 g steel sample dissolved in 50.00 mL of an acidic solution was determined by tiration with a standardized 0.100 M potassium permanganate ( KMnO 4 , 158.034 g/mol) solution. The titration required 43.16 mL to reach the end point. What is the concentration of iron in the steel sample? Express your answer as grams of Fe per gram of steel. MnO − 4 + 8 H + + 5 Fe 2 + − ⇀ ↽ − Mn 2 + + 5 Fe 3 + + 4 H 2 O

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 07:30
Which of the following best supports the concept that genetic information is passed on to offspring from both of their parents, not just one?
Answers: 2

Chemistry, 22.06.2019 12:00
An atom's configuration based on its number of electrons ends at 3p4. another atom has seven more electrons. starting at 3p, what is the remaining configuration? 3p63d34s2 3p43d54s2 3p64s23d3 3p44s23d
Answers: 3

Chemistry, 23.06.2019 09:00
Chortling is used to clean water. another possible atom that would also work is a. sodium b. sulfur c. bromine
Answers: 1
You know the right answer?
The Fe 2 + ( 55.845 g/mol) content of a 2.349 g steel sample dissolved in 50.00 mL of an acidic solu...
Questions




Computers and Technology, 12.07.2019 09:30


English, 12.07.2019 09:30

History, 12.07.2019 09:30

History, 12.07.2019 09:30



Business, 12.07.2019 09:30



Mathematics, 12.07.2019 09:30


History, 12.07.2019 09:30





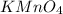 solution = 0.100 M
solution = 0.100 M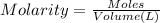
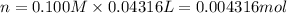
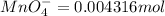
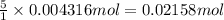 of ferrous ions
of ferrous ions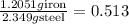 iron per gram of steel
iron per gram of steel


