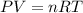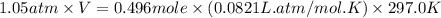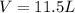Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 02:00
If you add 10ml of hot water to 10ml of cold water and the change in tempature 8°c calculate how much energy is gained by the cold water
Answers: 1

Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical solutions?
Answers: 1

Chemistry, 22.06.2019 23:00
Consider the reaction: 2al(s) + fe2o3(s) → al2o3(s) + 2fe(s) the δhf for fe2o3(s) = -824.3 kj/mole. the δhf for al2o3(s) = -1675.7 kj/mole. finish the equation. δhrxn = [(1)( kj/mole) + (2)( kj/mole)] - [(1)( kj/mole) + (2) ( kj/mole)]
Answers: 1

Chemistry, 23.06.2019 03:30
The molar mass of iron(fe) is 55.8 g/mol. what is the mass in grams of 2.25 moles of iron?
Answers: 1
You know the right answer?
Ammonium carbonate decomposes upon heating according to the following balanced equation:
(NH4)...
(NH4)...
Questions


Chemistry, 12.01.2021 04:10

Health, 12.01.2021 04:10




Social Studies, 12.01.2021 04:10




Arts, 12.01.2021 04:10


Mathematics, 12.01.2021 04:10

History, 12.01.2021 04:10

Mathematics, 12.01.2021 04:10


Computers and Technology, 12.01.2021 04:10




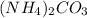 = 96.094 g/mol
= 96.094 g/mol
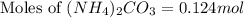
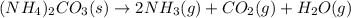
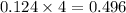 mole of gas
mole of gas