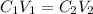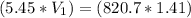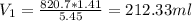8. What volume (ml) of a 5.45 M lead nitrate solution must be diluted to 820.7 ml to make a
1....

Chemistry, 21.02.2020 05:13 brandiwingard
8. What volume (ml) of a 5.45 M lead nitrate solution must be diluted to 820.7 ml to make a
1.41 M solution of lead nitrate?
A) 212
B) 6310
C) 3170
D) 0.00936
E) 0.00471
A
.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:00
Different isotopes indicate that an element will have different numbers of
Answers: 2

Chemistry, 22.06.2019 15:00
Many ionic compounds and a few highly polar covalent compounds are because they completely ionize in water to create a solution filled with charged ions that can conduct an electric current.
Answers: 1

Chemistry, 22.06.2019 16:40
Identify the lewis acid in this balanced equation: ag+ + 2nh3 -> ag(nh3)2+a. ag+b. nh3c. ag(nh3)2+
Answers: 1

Chemistry, 22.06.2019 19:30
Chlorine and water react to form hydrogen chloride and oxygen, like this: 2cl2 (g) + 2h2o (g) → 4hcl (g) + o2 (g) also, a chemist finds that at a certain temperature the equilibrium mixture of chlorine, water, hydrogen chloride, and oxygen has the following composition: compound concentration at equilibrium cl2 0.55m h2o 0.57m hcl 0.53m o2 0.34m calculate the value of the equilibrium constant kc for this reaction. round your answer to 2 significant digits.
Answers: 2
You know the right answer?
Questions


Mathematics, 06.01.2021 16:40


Mathematics, 06.01.2021 16:40


Mathematics, 06.01.2021 16:40

English, 06.01.2021 16:40

English, 06.01.2021 16:40



History, 06.01.2021 16:40




English, 06.01.2021 16:40


History, 06.01.2021 16:40

Mathematics, 06.01.2021 16:40

English, 06.01.2021 16:40