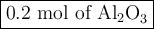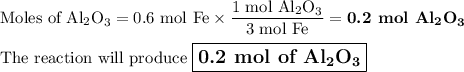Questlon 22
U.1 pts
Given the following equation:
2Al(s) +3 FeO(s)
3Fe(s)+ A...


Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 18:00
Does anyone know a lot about how to: - calculate mass of magnesium metal - calculate the actual yield of magnesium oxide - calculate the theoretical yield of mgo - calculate the percent yield of mgo - determine the percent yield of mgo - determine the average percent yield of mgo i had to do an online lab and its asking these questions but i have no idea where to start or how to be able to find these things. i can post the chart of the data from the lab or if you can tell me exactly how i can find each.
Answers: 3

Chemistry, 21.06.2019 19:20
What is the strongest intermolecular force between an nacl unit and an h2o molecule together in a solution? covalent bonding dipole-dipole force hydrogen bonding ion-dipole force
Answers: 1

Chemistry, 21.06.2019 23:10
Nitrogen (n), phosphorus (p), and potassium (k) are the main nutrients in plant fertilizers. according to an industry convention, the numbers on the label refer to the mass percents of n, p2o5, and k2o, in that order. calculate the n: p: k ratio of a 30: 10: 10 fertilizer in terms of moles of each element, and express it as x: y: 1.0.
Answers: 1

Chemistry, 22.06.2019 10:00
Water's surface tension and heat storage capacity are accounted for by its a) orbitals b) weight c) hydrogen bonds d) mass e) size
Answers: 2
You know the right answer?
Questions


Health, 08.10.2019 07:20

Social Studies, 08.10.2019 07:30



English, 08.10.2019 07:30


Mathematics, 08.10.2019 07:30

Business, 08.10.2019 07:30

Mathematics, 08.10.2019 07:30

History, 08.10.2019 07:30

Mathematics, 08.10.2019 07:30





Mathematics, 08.10.2019 07:30

Social Studies, 08.10.2019 07:30

Biology, 08.10.2019 07:30

History, 08.10.2019 07:30