
Chemistry, 21.02.2020 05:29 Totototono
"Compounds A and B react to give a single product, C. Write the rate law for each of the following cases and determine the units of the rate constant by using the units M for concentration and s for time.
a. The reaction is first order in A and second order in B.
b. The reaction is first order in A and second order overall.
c. The reaction is independent of the concentration of A and second order overall.
d. The reaction is second order in both A and B."

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:30
How many moles are in 250 grams of tungsten (w)? * 4.4x10^23 moles 4.2x10^23 moles 0.7 moles 1.4 moles
Answers: 3

Chemistry, 21.06.2019 23:00
An electrons position cannot be known precisely only it's probability of being in a certain location can be known
Answers: 1

Chemistry, 22.06.2019 09:00
Look at the spectrums of a star moving towards earth and a motionless star. which of these is a correct inference that can be draw from the observation of the two spectrums? (2 points) the spectrum of a motionless star is difficult to be viewed separately using oridinary telescopes. the spectrum of a motionless star is identical to the spectrum of a star which moves towards earth. the spectrum of a star shifts towards the red region when the star moves towards earth. the spectrum of a star shifts towards the blue region when the star moves towards earth.
Answers: 2

You know the right answer?
"Compounds A and B react to give a single product, C. Write the rate law for each of the following c...
Questions


Mathematics, 02.03.2020 06:40


Physics, 02.03.2020 06:41

Mathematics, 02.03.2020 06:41

Mathematics, 02.03.2020 06:41

Mathematics, 02.03.2020 06:41

Chemistry, 02.03.2020 06:41



Mathematics, 02.03.2020 06:41



History, 02.03.2020 06:42

Mathematics, 02.03.2020 06:42



Health, 02.03.2020 06:44

Mathematics, 02.03.2020 06:44

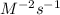 where reaction is first order in A and second order in B
where reaction is first order in A and second order in B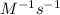 where reaction is first order in A and second order overall.
where reaction is first order in A and second order overall.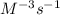 where reaction reaction is second order in both A and B.
where reaction reaction is second order in both A and B.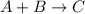
![r=k[A]^x[B]^y](/tpl/images/0518/9509/dc73f.png)
![r=k[A]^1[B]^2](/tpl/images/0518/9509/8b1e6.png)
![r=k[A]^1[B]^2\\M/s =k[M]^1[M]^2\\M/s =k[M]^{1+2}\\M/s =k[M]^{3}\\M^{1-3}/s =k\\M^{-2}s^{-1} =k](/tpl/images/0518/9509/cc600.png)
![r=k[A]^1[B]^1](/tpl/images/0518/9509/76928.png)
![r=k[A]^1[B]^1\\M/s =k[M]^1[M]^1\\M/s =k[M]^{1+1}\\M/s =k[M]^{2}\\M^{1-2}/s =k\\M^{-1}s^{-1} =k](/tpl/images/0518/9509/93419.png)
![r=k[A]^0[B]^2](/tpl/images/0518/9509/19b06.png)
![r=k[B]^2\\M/s =k[M]^2\\M/s =k[M]^{2}\\M^{1-2}/s =k\\M^{-1}s^{-1} =k](/tpl/images/0518/9509/d1c9d.png)
![r=k[A]^2[B]^2](/tpl/images/0518/9509/33731.png)
![r=k[A]^2[B]^2\\M/s =k[M]^2[M]^2\\M/s =k[M]^{2+2}\\M/s =k[M]^{4}\\M^{1-4}/s =k\\M^{-3}s^{-1} =k](/tpl/images/0518/9509/06ac7.png)


