
Chemistry, 21.02.2020 05:28 cjtambasco
"Magnesium has three naturally occurring isotopes: A. Mg-24 with mass 24.3050 amu and a natural abundance of 78.99 %, B. Mg-25 with mass 24.9858 amu and a natural abundance of 10.00 %, and C. Mg-26 with mass 25.9826 amu and a natural abundance of 11.01 %."

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:30
What is the mass of each element in a 324.8 sample of co2
Answers: 1

Chemistry, 22.06.2019 08:30
Sally is making a model of a magnesium atom with an atomic mass number of 24 for her chemistry class. she has foam balls for the protons, neutrons, and electrons. she has added 6 neutrons to her model so far. how many more neutrons does she need to add to complete her neutral atom of magnesium?
Answers: 1

Chemistry, 22.06.2019 11:30
Which statement best describes the flow of energy in this scenario
Answers: 1

Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical systems? a) water dissolves nonpolar ionic compounds. b) water dissociates ionic compounds. c) water dissociates covalent molecules. d) water dissolves nonpolar covalent substances.
Answers: 1
You know the right answer?
"Magnesium has three naturally occurring isotopes: A. Mg-24 with mass 24.3050 amu and a natural abun...
Questions


Biology, 20.08.2019 10:30

Mathematics, 20.08.2019 10:30


Mathematics, 20.08.2019 10:30

Mathematics, 20.08.2019 10:30


Mathematics, 20.08.2019 10:30

Biology, 20.08.2019 10:30

Mathematics, 20.08.2019 10:30

English, 20.08.2019 10:30

Mathematics, 20.08.2019 10:30

Biology, 20.08.2019 10:30



English, 20.08.2019 10:30




Mathematics, 20.08.2019 10:30

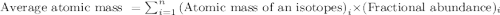 .....(1)
.....(1)![\text{Average atomic mass of magnesium}=[(24.3050\times 0.7899)+(24.9858\times 0.1000)+(25.9826\times 0.1101)]\\\\\text{Average atomic mass of magnesium}=24.5578amu](/tpl/images/0518/9477/add94.png)



