
Chemistry, 21.02.2020 05:56 swordnewsnetwork
A 71.0 mL 71.0 mL aliquot of a 1.30 M 1.30 M solution is diluted to a total volume of 248 mL. 248 mL. A 124 mL 124 mL portion of that solution is diluted by adding 133 mL 133 mL of water. What is the final concentration? Assume the volumes are additive.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:00
Drag each label to the correct location on the chart. classify each reaction as endothermic or exothermic.
Answers: 1

Chemistry, 22.06.2019 09:00
Astudent is asked to identify and element that is pale yellow brittle solid and does not conduct electricity. at which location in this periodic table would the element most likely be found?
Answers: 2

Chemistry, 22.06.2019 13:30
Which is true of a liquid? it has a definite volume but not a definite mass.it has a definite mass but not a definite volume.it has a definite volume but not a definite shape.it has a definite shape but not a definite volume.
Answers: 2

Chemistry, 23.06.2019 03:00
You have a sample of a metal, the sample is exactly 6.02 x 1023atom, if the sample has a mass 55.85 what metal is your sample made of?
Answers: 2
You know the right answer?
A 71.0 mL 71.0 mL aliquot of a 1.30 M 1.30 M solution is diluted to a total volume of 248 mL. 248 mL...
Questions

World Languages, 07.07.2021 19:10


Mathematics, 07.07.2021 19:10




Mathematics, 07.07.2021 19:10


Biology, 07.07.2021 19:10



Mathematics, 07.07.2021 19:10

Physics, 07.07.2021 19:10

Mathematics, 07.07.2021 19:10





History, 07.07.2021 19:10

Mathematics, 07.07.2021 19:10

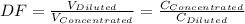
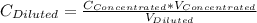 = 1.3×71/248 = 0.372 M
= 1.3×71/248 = 0.372 M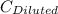 = concentration of new solution
= concentration of new solution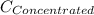 = 0.372 M
= 0.372 M 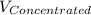 = 124 mL
= 124 mL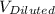 = 257 mL
= 257 mL

