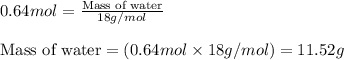Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 16:50
Which of the following is an indication that a substance has undergone a chemical change? a. no new product has been formed. b. the color of the substance has not changed. c. the original constitute has not changed. d. the molecular structure has changed.
Answers: 1

Chemistry, 22.06.2019 18:20
Which reason best explains why metals are malleable? a)because they have delocalized electrons b)because they have localized electrons c)because they have ionic bonds d)because they have rigid bonds
Answers: 2

Chemistry, 23.06.2019 00:00
Predict the relative bond lengths of the three carbon-oxygen bonds in the carbonate ion (co2−3). what would you expect the charge to be on each oxygen? match the words in the left column to the appropriate blanks in the sentences on the right. make certain each sentence is complete before submitting your answer.
Answers: 3
You know the right answer?
Trisilane (Si3H8) is a liquid with a density of 0.739 g cm-3. It reacts with oxygen to give silicon...
Questions

Physics, 24.03.2020 01:39




Mathematics, 24.03.2020 01:39

Mathematics, 24.03.2020 01:39

Mathematics, 24.03.2020 01:39

Mathematics, 24.03.2020 01:39


Mathematics, 24.03.2020 01:39


Biology, 24.03.2020 01:39

Social Studies, 24.03.2020 01:39


Mathematics, 24.03.2020 01:39




Chemistry, 24.03.2020 01:39

Mathematics, 24.03.2020 01:39

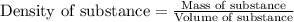
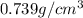
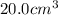
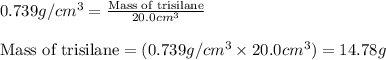
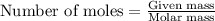 .....(1)
.....(1)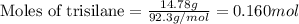
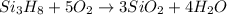
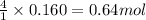 of water
of water