
Chemistry, 21.02.2020 17:42 macylen3900
Air bags are activated when a severe impact causes a steel ball to compress a spring and electrically ignite a detonator cap. This action causes sodium azide (NaN3) to decompose explosively according to the following reaction. 2 NaN3(s) → 2 Na(s) + 3 N2(g) What mass of NaN3(s) must be reacted to inflate an air bag to 70.6 L at STP?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 23:00
When determining the shape of a molecule, it is important to draw a lewis dot structure first in order to see the total number a. electrons within the moleculeb. bonding and unshared pairs around central atomc. unshared pair within the molecule( i really need it )
Answers: 1

Chemistry, 22.06.2019 19:30
Astring vibrates with a frequency of 10 hz. why can't a person hear the sound waves produced by the vibrating string, no matter how large the amplitude of the waves? out! this is homework and due tomorrow! you so much!
Answers: 2


Chemistry, 23.06.2019 13:20
Aluminum reacts with sulfuric acid to produce aluminum sulfate and hydrogen gas. how many grams of aluminum sulfate would be formed if 250 g h 2 so 4 completely reacted with aluminum? 2al( s ) + 3h 2 so 4 ( aq ) ? al 2 (so 4 ) 3 ( aq ) + 3h 2 ( g )
Answers: 1
You know the right answer?
Air bags are activated when a severe impact causes a steel ball to compress a spring and electricall...
Questions


Mathematics, 11.12.2020 03:10

English, 11.12.2020 03:10

Mathematics, 11.12.2020 03:10



Mathematics, 11.12.2020 03:10

Mathematics, 11.12.2020 03:10


Chemistry, 11.12.2020 03:10






Mathematics, 11.12.2020 03:10

Physics, 11.12.2020 03:10


Mathematics, 11.12.2020 03:10

Mathematics, 11.12.2020 03:10

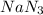 must be reacted to inflate an air bag to 70.6 L at STP.
must be reacted to inflate an air bag to 70.6 L at STP.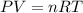
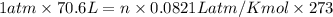
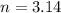
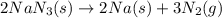
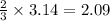 moles of
moles of 

