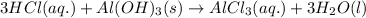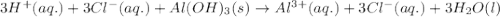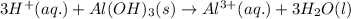Maalox and Mylanta are both antacids that contain aluminum hydroxide as their active ingredient. Write the balanced equation for the neutralization of hydrochloric acid with aluminum hydroxide, Al(OH)3. Include physical states. reaction: | 3HCl(g) + Al(OH)3(s) —— AlCl3(aq) + 3H2O(1) Identify the spectator ions in this reaction. OOH OH A13+ ci-

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 04:30
Long term exposure to waves can cause sunburns and skin cancer. a) visible b) infrared c) gamma rays d) ultraviole
Answers: 1

Chemistry, 22.06.2019 18:00
How is energy related to the change of state represented by the model? atoms gain energy as a solid changes to a liquid. atoms gain energy as a solid changes to a gas. atoms lose energy as a solid changes to a liquid. atoms lose energy as a solid changes to a gas.
Answers: 3

Chemistry, 22.06.2019 18:30
Which rate indicates the number of children that would be born per woman if she were to live to the end of her child bearing years
Answers: 2
You know the right answer?
Maalox and Mylanta are both antacids that contain aluminum hydroxide as their active ingredient. Wri...
Questions


History, 01.11.2019 11:31

Mathematics, 01.11.2019 11:31



History, 01.11.2019 11:31

Mathematics, 01.11.2019 11:31


Mathematics, 01.11.2019 11:31

Biology, 01.11.2019 11:31



English, 01.11.2019 11:31


History, 01.11.2019 11:31

Social Studies, 01.11.2019 11:31