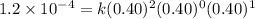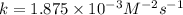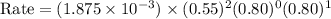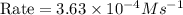Chemistry, 21.02.2020 19:53 faithtunison
For the reaction A+B+C→D+E, the initial reaction rate was measured for various initial concentrations of reactants. The following data were collected:
Trial [A]
(M) [B]
(M) [C]
(M) Initial rate
(M/s)
1 0.40 0.40 0.40 1.2×10−4
2 0.40 0.40 1.20 3.6×10−4
3 0.80 0.40 0.40 4.8×10−4
4 0.80 0.80 0.40 4.8×10−4
Given the data calculated in Parts A, B, C, and D, determine the initial rate for a reaction that starts with 0.55 M of reagent A and 0.80 M of reagents B and C?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:30
This question is about electrolysis. metal spoons can be coated with silver. this is called electroplating. suggest one reason why spoons are electroplated?
Answers: 1

Chemistry, 22.06.2019 04:30
The big bang nucleosynthesis theory states that elements were produced in the first few minutes of the big bang while elements have their origins in the interiors of stars, forming much later in the history of the universe.
Answers: 1

Chemistry, 22.06.2019 12:20
Which is an example of the practical pursuit of alchemy? a. forming perfect substances. b. transforming base metals. c. developing metalworking techniques. d. linking spiritual characteristics with material substances.
Answers: 1

Chemistry, 23.06.2019 05:00
110 g of water (specific heat = 4.184 j/g c) and 100 g of a metal sample (specific heat = 0.397 j/g c) are heated from 25 degrees c to 75 degrees c. which substance required more thermal energy?
Answers: 1
You know the right answer?
For the reaction A+B+C→D+E, the initial reaction rate was measured for various initial concentration...
Questions

History, 17.08.2020 17:01


Mathematics, 17.08.2020 17:01


English, 17.08.2020 18:01

Mathematics, 17.08.2020 18:01

Mathematics, 17.08.2020 18:01



Mathematics, 17.08.2020 18:01





English, 17.08.2020 18:01


Mathematics, 17.08.2020 18:01

Biology, 17.08.2020 18:01


Mathematics, 17.08.2020 18:01

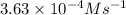
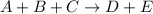
![\text{Rate}=k[A]^a[B]^b[C]^c](/tpl/images/0519/4605/be89a.png)
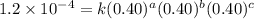 ....(1)
....(1)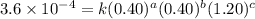 ....(2)
....(2)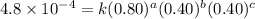 ....(3)
....(3)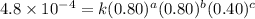 ....(4)
....(4)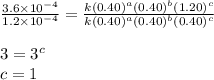
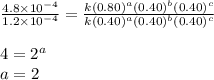
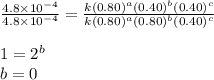
![\text{Rate}=k[A]^2[B]^0[C]^1](/tpl/images/0519/4605/54afd.png)