
Electron transfer is the term for this process. The resulting anion and cation are attracted by Coulombic forces and an ionic compound is formed. Ionic compounds are neutral: the number of electrons lost in forming the cation(s) must equal the number gained in forming the anion(s). For example, Aluminum (Al, Z = 13, Group 3A or 13) will lose 3 electrons to become Al3+ which is isoelectronic with Neon (Ne), its closest noble gas. To form an ionic compound those 3 electrons must be picked up by a nonmetal. Two possibilities are:
Nitrogen (N, Z = 7, Group 5A or 15) gains all 3 electrons to become isoelectronic with Neon (Z = 10). The compound formed is AlN
3 Chlorine atoms (Cl, Z = 17, Group 7A or 17) each gain 1 electron to become isoelectronic with Argon (Ar, Z = 18). The symbol for the compound formed is AlCl3. The subscript after Cl indicates that 3 Cl- anions are required.
(1 pt) Form an ionic compound with sodium (Na):
Its atomic number is:
How many electrons must it lose to have the same number of electrons as its nearest noble gas?
Write the symbol for the ion:
Which nonmetal atom needs to gain that same number of electrons to have the same number of electrons as its nearest noble gas?
Write the symbol for the compound formed:
(1 pt) Form an ionic compound with calcium (Ca):
Its atomic number is:
How many electrons must it lose to have the same number of electrons as its nearest noble gas?
Write the symbol for the ion:
Which nonmetal atom needs to gain that same number of electrons to have the same number of electrons as its nearest noble gas?
Write the symbol for the compound formed:

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 01:30
100 points answer quick the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1


Chemistry, 23.06.2019 07:00
Which of the following statements is true? an atom consists of protons, electrons, and neutrons.an atom consists of protons and neutrons.an atom consists of electrons bonded to one another.an atom consists of protons bonded to one another.
Answers: 1

Chemistry, 23.06.2019 09:00
What factor besides temperature affects the boiling point of water? a. mass b. number of moles c. volume d. pressure
Answers: 3
You know the right answer?
Electron transfer is the term for this process. The resulting anion and cation are attracted by Coul...
Questions



Mathematics, 03.11.2019 07:31

Physics, 03.11.2019 07:31




Chemistry, 03.11.2019 07:31

English, 03.11.2019 07:31

English, 03.11.2019 07:31



History, 03.11.2019 07:31




Mathematics, 03.11.2019 07:31


Mathematics, 03.11.2019 07:31

History, 03.11.2019 07:31

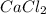
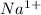 which is isoelectronic to Neon (Ne ,Z = 10).The symbol for the sodium ion is
which is isoelectronic to Neon (Ne ,Z = 10).The symbol for the sodium ion is 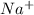 .Chlorine atom (
.Chlorine atom (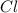 , Z = 17, Group 7A or 17) gains 1 elecron to be isoelectronic to neon.The symbol for the compound formed is NaCl.PART 2:Atomic number of calcium is 20Calcium(Ca, Z = 20 ,Group 2A) will lose two electrons to become
, Z = 17, Group 7A or 17) gains 1 elecron to be isoelectronic to neon.The symbol for the compound formed is NaCl.PART 2:Atomic number of calcium is 20Calcium(Ca, Z = 20 ,Group 2A) will lose two electrons to become 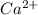 which is isoelectronic to argon. The symbol for the ion is
which is isoelectronic to argon. The symbol for the ion is 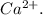 Two chlorine atoms (Cl, Z = 17, Group 7A or 17) each gains one electron to be isoelectronic to argon(Z = 18)The symbol for the compound formed is
Two chlorine atoms (Cl, Z = 17, Group 7A or 17) each gains one electron to be isoelectronic to argon(Z = 18)The symbol for the compound formed is 

