Chemistry, 21.02.2020 21:57 usagimiller
The standard enthalpies of combustion of cis-2-hexene and trans-2-hexene (to form carbon dioxide and water) are −3727.9 kJ·mol−1 and −3726.3 kJ·mol−1, respectively. Calculate the enthalpy of the following isomerization process.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.036m naoh best answer will be brainliest
Answers: 3

Chemistry, 22.06.2019 19:40
Scientists have developed an explanation of a phenomenon from several verified hypotheses. the explanation has been confirmed through numerous experimental tests.which option best describes this explanation? a. scientific lawb. research questionc. hypothesisd. scientific theory
Answers: 3

Chemistry, 22.06.2019 20:00
What is the molarity of the solution produced when 145 g of nacl is dissolved in sufficient water to prepare 2.75 l of solution?
Answers: 1

Chemistry, 22.06.2019 20:30
Identify the correct mole ratio for each substance. sodium chloride (nacl) na: cl = 1: ammonium nitrate (nhno) h: o = 4:
Answers: 1
You know the right answer?
The standard enthalpies of combustion of cis-2-hexene and trans-2-hexene (to form carbon dioxide and...
Questions

Arts, 10.12.2020 01:10



Mathematics, 10.12.2020 01:10

Mathematics, 10.12.2020 01:10

Geography, 10.12.2020 01:10

Mathematics, 10.12.2020 01:10


Mathematics, 10.12.2020 01:10

Mathematics, 10.12.2020 01:10



Mathematics, 10.12.2020 01:10




Mathematics, 10.12.2020 01:10


History, 10.12.2020 01:10

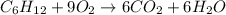
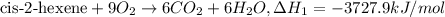 ..[1]
..[1]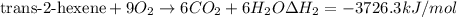 [2]
[2]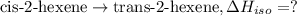 ...[3]
...[3]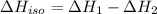 (Hess's law)
(Hess's law)