Chemistry, 21.02.2020 21:53 jalenshayewilliams
5. The gas-phase decomposition of ethyl iodide to give ethylene and hydrogen iodide is a first-order reaction. C2H5I C2H4 + HI At 600 K, the value of k is 1.60 × 10– 5 s– 1. When the temperature is raised to 700 K, the value of k increases to 6.36 × 10– 3 s– 1. What is the activation energy for this reaction?

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 20:30
Which states of matter have particles that move independently of one another with very little attraction?
Answers: 1


Chemistry, 23.06.2019 05:30
Awhite powder is added to a solution. the images show observations made before the powder is added, just after the powder has been added, and a little while later. (the liquid in the small beaker is phenol red solution.) what evidence shows that a chemical change has taken place?
Answers: 1
You know the right answer?
5. The gas-phase decomposition of ethyl iodide to give ethylene and hydrogen iodide is a first-order...
Questions

English, 11.10.2020 14:01

Mathematics, 11.10.2020 14:01

English, 11.10.2020 14:01


Mathematics, 11.10.2020 14:01

English, 11.10.2020 14:01

Biology, 11.10.2020 14:01


Mathematics, 11.10.2020 14:01


Mathematics, 11.10.2020 14:01

Biology, 11.10.2020 14:01


English, 11.10.2020 14:01

Mathematics, 11.10.2020 14:01


Mathematics, 11.10.2020 14:01


Mathematics, 11.10.2020 14:01

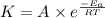
![\log (\frac{K_2}{K_1})=\frac{E_a}{2.303\times R}[\frac{1}{T_1}-\frac{1}{T_2}]](/tpl/images/0519/6420/1504e.png)
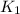 = rate constant at
= rate constant at 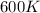 =
= 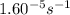
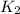 = rate constant at
= rate constant at 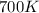 =
= 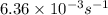
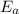 = activation energy for the reaction = ?
= activation energy for the reaction = ?
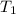 = initial temperature =
= initial temperature = 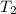 = final temperature =
= final temperature = ![\log (\frac{6.36\times 10^{-3}s^{-1}}{1.60\times 10^{-5}s^{-1}})=\frac{E_a}{2.303\times 8.314J/mole.K}[\frac{1}{600K}-\frac{1}{700K}]](/tpl/images/0519/6420/54c04.png)
![2.60=\frac{E_a}{2.303\times 8.314J/mole.K}[\frac{1}{600K}-\frac{1}{700K}]](/tpl/images/0519/6420/52269.png)