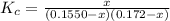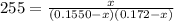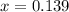Chemistry, 21.02.2020 21:58 leahstubbs
For the following reaction, Kc = 255 at 1000 K. CO(g)+Cl2(g)⇌COCl2(g) A reaction mixture initially contains a CO concentration of 0.1550 M and a Cl2 concentration of 0.172 M at 1000 K.
What is the equilibrium concentration of CO at 1000 K?
What is the equilibrium concentration of Cl2 at 1000 K?
What is the equilibrium concentration of COCl2 at 1000 K?

Answers: 3
Another question on Chemistry



Chemistry, 22.06.2019 16:30
How many moles of sulfuric acid (h2so4) are needed to react completely with 6.8 moles of lithium hydroxide (lioh)? 2lioh + h2so4 → li2so4 + 2h2o a. 3.4 mol h2so4b. 6.8 mol h2so4 c. 10.2 mol h2so4 d. 13.6 mol h2so4
Answers: 3

Chemistry, 22.06.2019 20:30
Some familiar products contain some of the same types of atoms. for instance, the chemical formula for baking soda is nahco 3. the chemical formula for liquid bleach is naclo, and the chemical formula for table salt is nacl. which choice best describes why these three products have some of the same types of atoms in common?
Answers: 1
You know the right answer?
For the following reaction, Kc = 255 at 1000 K. CO(g)+Cl2(g)⇌COCl2(g) A reaction mixture initially c...
Questions


Mathematics, 02.12.2020 20:50


Mathematics, 02.12.2020 20:50

Mathematics, 02.12.2020 20:50

English, 02.12.2020 20:50


History, 02.12.2020 20:50

Mathematics, 02.12.2020 20:50


Mathematics, 02.12.2020 20:50


History, 02.12.2020 20:50

Spanish, 02.12.2020 20:50

Mathematics, 02.12.2020 20:50



Social Studies, 02.12.2020 20:50


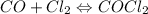
 initial
initial![K_c = \frac{[COCl_2]}{[CO][Cl_2]}](/tpl/images/0519/6554/f9140.png)
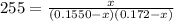
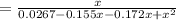
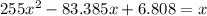
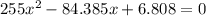
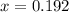
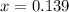 (correct value)
(correct value)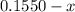
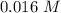
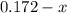
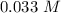

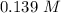
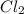 at 1000 K is= 0.033 M and the equilibrium concentration of
at 1000 K is= 0.033 M and the equilibrium concentration of 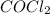 at 1000 K is 0.139 M
at 1000 K is 0.139 M 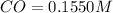
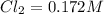
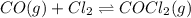
![K_c=\frac{[COCl_2]}{[CO][Cl_2]}](/tpl/images/0519/6554/36d91.png)