Chemistry, 21.02.2020 22:44 princessammarah4731
When 0.49 g of a molecular compound was dissolved in 20.00 g of cyclohexane, the freezing point of the solution was lowered by 3.9 0C. Determine the molecular mass of this compound.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 08:30
Which common material is an example of a polymer? (25 pts) a. steel b. plastic c. petroleum d. rubbing alcohol
Answers: 2


Chemistry, 22.06.2019 20:30
The activation energy for the reaction no2(g)+co2(g)⟶no(g)+co(g) is ea = 300 kj/mol and the change in enthalpy for the reaction is δh = -100 kj/mol . what is the activation energy for the reverse reaction?
Answers: 3

Chemistry, 22.06.2019 23:30
Rank substituents in order of their priority when assigning the e or z label to an alkene. i, ch2i , h, ch2ch2cl, f
Answers: 2
You know the right answer?
When 0.49 g of a molecular compound was dissolved in 20.00 g of cyclohexane, the freezing point of t...
Questions



Mathematics, 01.07.2019 18:30


History, 01.07.2019 18:30


Mathematics, 01.07.2019 18:30




Chemistry, 01.07.2019 18:30

Chemistry, 01.07.2019 18:30






Chemistry, 01.07.2019 18:30

Chemistry, 01.07.2019 18:30

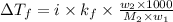
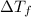 = depression in freezing point =
= depression in freezing point = 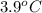
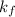 = freezing point constant =
= freezing point constant = 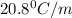
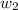 = mass of solute = 0.49 g
= mass of solute = 0.49 g
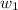 = mass of solvent (cyclohexane) = 20.00 g
= mass of solvent (cyclohexane) = 20.00 g
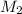 = molar mass of solute = ?
= molar mass of solute = ?