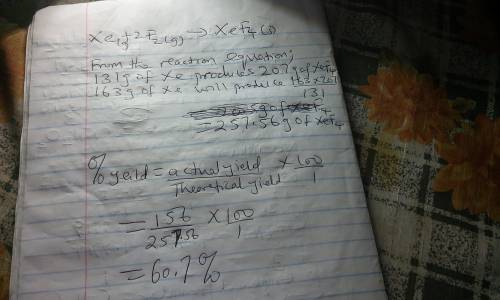Chemistry, 22.02.2020 01:45 cjtambasco
Although they were formerly called the inert gases, at least the heavier elements of Group 8 do form relatively stable compounds. For example, xenon combines directly with elemental fluorine at elevated temperatures in the presence of a nickel catalyst. Use table 1 and table 2. Xe(g) + 2 F2(g) → XeF4(s) What is the theoretical mass of xenon tetrafluoride that should form when 163 g of xenon is reacted with 164 g of F2? g= What is the percent yield if only 156 g of XeF4 is actually isolated?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 21:00
Of the groups of elements below, which are most likely to gain electrons to become anions? a. alkali metal b. boron group c. halogen d. transition metal
Answers: 2

Chemistry, 23.06.2019 06:30
Acertain atom has 22 protons and 19 electrons. this atom loses an electron. the net charge on the atom is now 4+1+01-4-. if this same atom with 22 protons and 19 electrons were to gain 3 electrons, the net charge on the atom would be 3+2+02-3-.
Answers: 1

Chemistry, 23.06.2019 09:00
Which factor is likely to impact the possible number of compounds? presence of unlimited number of elements in the periodic table the inability of atoms to align perfectly with other atoms the ability of all elements to react with every other element all elements being equally reactive
Answers: 2

You know the right answer?
Although they were formerly called the inert gases, at least the heavier elements of Group 8 do form...
Questions

Advanced Placement (AP), 06.07.2019 23:30

Business, 06.07.2019 23:30

Physics, 06.07.2019 23:30


Chemistry, 06.07.2019 23:30


Advanced Placement (AP), 06.07.2019 23:30


Biology, 06.07.2019 23:30

History, 06.07.2019 23:30



Mathematics, 06.07.2019 23:30


Mathematics, 06.07.2019 23:30


Mathematics, 06.07.2019 23:30

Biology, 06.07.2019 23:30


English, 06.07.2019 23:30