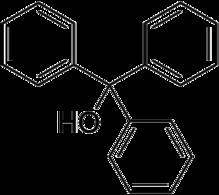
Chemistry, 22.02.2020 04:16 viridianasar5158
After addition of the benzophenone solution to the Grignard reagent, you realize that you mistakenly used diethyl ether from the ether for workup bottle (NOT the correct anhydrous ether bottle) to make the benzophenone solution. Draw the chemical structure of the organic product that was formed from a side reaction (NOT the desired reaction) that likely occurred in your reaction vial.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 03:50
Consider the reaction: n2(g) + o2(g) ? 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 06:30
Particle model to predict what will happen if a sharp object creates a hole in the soccer ball
Answers: 2


Chemistry, 22.06.2019 14:30
100 grams of molten lead (600°c) is used to make musket balls. if the lead shot is allowed to cool to room temperature (21°c), what is the change in entropy (in j/k) of the lead? (for the specific heat of molten and solid lead use 1.29 j/g⋅°c; the latent heat of fusion and the melting point of lead are 2.45 × 104 j/kg and 327°c, respectively.)
Answers: 1
You know the right answer?
After addition of the benzophenone solution to the Grignard reagent, you realize that you mistakenly...
Questions



Computers and Technology, 28.06.2019 08:30

History, 28.06.2019 08:30

Biology, 28.06.2019 08:30

Physics, 28.06.2019 08:30


Mathematics, 28.06.2019 08:30

Mathematics, 28.06.2019 08:30