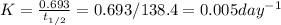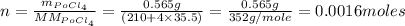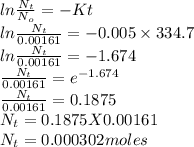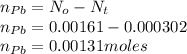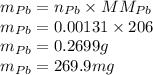Chemistry, 22.02.2020 06:17 antbanks3050
Polonium-210, 210Po, decays to lead-206, 206Pb, by alpha emission according to the equation
210 206 4
84 Po > 82Pb + 2 He
If the half-life, t1/2, of 210Po is 138.4 days, calculate the mass of 206Pb that can be produced from a 565.0-mg sample of polonium(IV) chloride, PoCl4, that is left untouched for 334.7 days. Assume that the only polonium isotope present in the sample is the 210Po isotope. The isotopic molar masses of 210Po is 209.98 g/mol and 206Pb is 205.97 g/mol.

Answers: 1
Another question on Chemistry

Chemistry, 23.06.2019 00:30
The footprints of a dinosaur and the burrow of an ancient shrimp are examples of which kind of fossils
Answers: 2

Chemistry, 23.06.2019 02:50
What is the typical rotational frequency frot for a molecule like n2 at room temperature (25∘c)? assume that d for this molecule is 1å=10−10m. take the total mass of an n2 molecule to be mn2=4.65×10−26kg. you will need to account for rotations around two axes (not just one) to find the correct frequency. express frot numerically in hertz, to three significant figures.
Answers: 3

Chemistry, 23.06.2019 05:30
The term gas is limited to those substances that exist in the gaseous state at
Answers: 1

Chemistry, 23.06.2019 06:00
Robert leaves a chocolate bar in his car while attending school all day. when he goes to his car in the afternoon, the bat has changed into gooey liquid. what happened to the chocolate bar
Answers: 1
You know the right answer?
Polonium-210, 210Po, decays to lead-206, 206Pb, by alpha emission according to the equation
Questions

Mathematics, 05.02.2020 09:56

Computers and Technology, 05.02.2020 09:56


History, 05.02.2020 09:56


Biology, 05.02.2020 09:56

Biology, 05.02.2020 09:56


Social Studies, 05.02.2020 09:56

Mathematics, 05.02.2020 09:56

Mathematics, 05.02.2020 09:56

Health, 05.02.2020 09:56

Mathematics, 05.02.2020 09:56






Mathematics, 05.02.2020 09:56