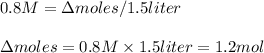Chemistry, 22.02.2020 18:57 kaleahearly123
Consider the following reaction:
2H2O2(aq)→2H2O(l)+O2(g)
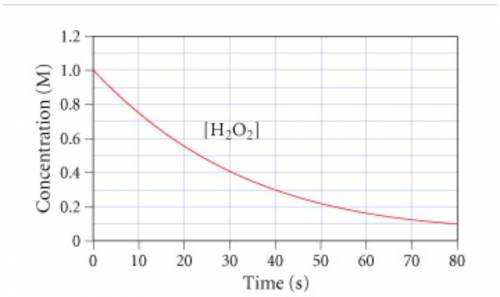
The graph (Figure 1) shows the concentration of H2O2 as a function of time.
If the instantaneous rate of formation of O2 is 3.3*(10^-3) moles/(liters*seconds), then...
If the initial volume of the H2O2 solution is 1.5 L , what total amount of O2 (in moles) is formed in the first 50 s of reaction?
Express your answer using two significant figures.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 08:30
The mass of a neutron is equal to the mass of a proton plus the mass of an electron. true or false false true
Answers: 1

Chemistry, 22.06.2019 09:30
Melissa is interested in her family tree and how her family has changed over its many generations. melissa probably more closely resembles
Answers: 2

Chemistry, 22.06.2019 11:50
Which of the following statements about hybrid orbitals is or are true? choose all that apply. choose all that apply. under sp2 hybridization, the large lobes point to the vertices of an equilateral triangle. after an atom undergoes sp hybridization there is one unhybridized p orbital on the atom. the angle between the large lobes of sp3 hybrids is 109.5∘
Answers: 2

Chemistry, 22.06.2019 14:30
According to le chatelier’s principle, a system in chemical equilibrium responds to stress by shifting the equilibrium in a direction that reduces the stress. normalizes the stress. increases the stress. changes the stress.
Answers: 1
You know the right answer?
Consider the following reaction:
2H2O2(aq)→2H2O(l)+O2(g)
The graph (Figure 1...
2H2O2(aq)→2H2O(l)+O2(g)
The graph (Figure 1...
Questions


Biology, 21.04.2021 15:30


History, 21.04.2021 15:30


Mathematics, 21.04.2021 15:30

Chemistry, 21.04.2021 15:30

Mathematics, 21.04.2021 15:30

Mathematics, 21.04.2021 15:30






Social Studies, 21.04.2021 15:30



History, 21.04.2021 15:30


Advanced Placement (AP), 21.04.2021 15:30

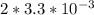
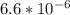
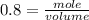
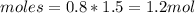
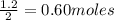 O₂ is formed in the first 50 s of reaction.
O₂ is formed in the first 50 s of reaction.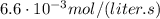
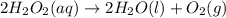
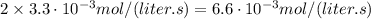
![\Delta [H_2O_2]=\Delta moles/Volume(liters)](/tpl/images/0520/3300/3d5f9.png)