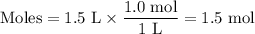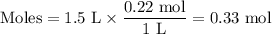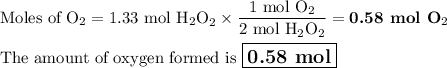Chemistry, 22.02.2020 19:58 MarishaTucker
Consider the following reaction:
2H2O2(aq)→2H2O(l)+O2(g)
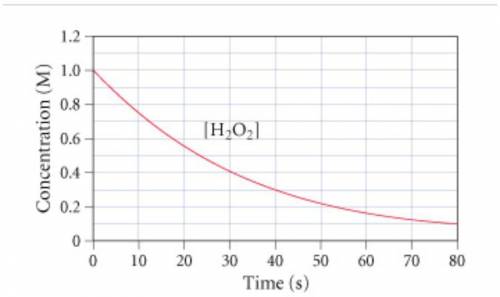
The graph (Figure 1) shows the concentration of H2O2 as a function of time.
If the instantaneous rate of formation of O2 is 3.3*(10^-3) moles/(liters*seconds), then...
If the initial volume of the H2O2 solution is 1.5 L, what total amount of O2 (in moles) is formed in the first 50 s of reaction?
Express your answer using two significant figures.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 15:30
Complete this brønsted-lowry reaction placing each product by its appropriate label. hso4- + hcn
Answers: 1

Chemistry, 22.06.2019 13:00
Is 9 correct? and can someone me with 10? it’s due tomorrow, you
Answers: 1

Chemistry, 22.06.2019 15:50
Elements in group 2 are all called alkaline earth metals. what is most similar about the alkaline earth metals?
Answers: 1

Chemistry, 22.06.2019 17:00
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 3
You know the right answer?
Consider the following reaction:
2H2O2(aq)→2H2O(l)+O2(g)
The graph (Figure 1) shows the...
2H2O2(aq)→2H2O(l)+O2(g)
The graph (Figure 1) shows the...
Questions



Arts, 07.09.2020 02:01


Mathematics, 07.09.2020 02:01

Mathematics, 07.09.2020 02:01

Health, 07.09.2020 02:01

Advanced Placement (AP), 07.09.2020 02:01



Mathematics, 07.09.2020 02:01

English, 07.09.2020 02:01




Mathematics, 07.09.2020 02:01

Mathematics, 07.09.2020 02:01

Social Studies, 07.09.2020 02:01


Mathematics, 07.09.2020 02:01