Consider the following reaction:
2H2O2(aq)→2H2O(l)+O2(g)
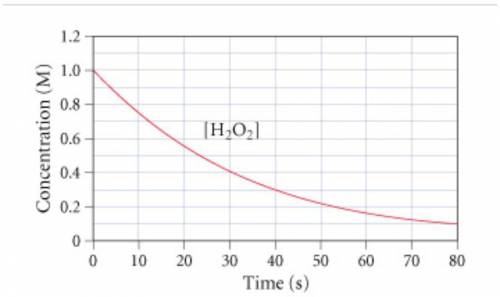
The graph (Figure 1...

Chemistry, 22.02.2020 20:03 beanokelley
Consider the following reaction:
2H2O2(aq)→2H2O(l)+O2(g)
The graph (Figure 1) shows the concentration of H2O2 as a function of time.
If the instantaneous rate of formation of O2 is 3.3*(10^-3) moles/(liters*seconds), then...
If the initial volume of the H2O2 solution is 1.5 L , what total amount of O2 (in moles) is formed in the first 50 s of reaction?
Express your answer using two significant figures.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 21:40
It is important to wear proper protective equipment in lab even when not actively performing experiments because accidents can affect any researcher, even one not working on an experiment. select the best answer from the choices provided
Answers: 3



Chemistry, 22.06.2019 09:30
Which element is the least metallic between cadmium, silver, zinc, or iron?
Answers: 1
You know the right answer?
Questions



Mathematics, 03.03.2021 17:40

Mathematics, 03.03.2021 17:40

Mathematics, 03.03.2021 17:40

Social Studies, 03.03.2021 17:40




Mathematics, 03.03.2021 17:40


Social Studies, 03.03.2021 17:40



Mathematics, 03.03.2021 17:40


Social Studies, 03.03.2021 17:40



Mathematics, 03.03.2021 17:40



